Exam 10: Introduction to Metabolism
Exam 1: Introduction to Biochemistry72 Questions
Exam 2: Water94 Questions
Exam 3: Amino Acids and the Primary Structures of Proteins107 Questions
Exam 4: Proteins: Three-Dimensional Structure and Function116 Questions
Exam 5: Properties of Enzymes91 Questions
Exam 6: Mechanisms of Enzymes88 Questions
Exam 7: Coenzymes and Vitamins93 Questions
Exam 8: Carbohydrates92 Questions
Exam 9: Lipids and Membranes95 Questions
Exam 10: Introduction to Metabolism87 Questions
Exam 11: Glycolysis88 Questions
Exam 12: Gluconeogenesis, the Pentose Phosphate Pathway, and Glycogen Metabolism90 Questions
Exam 13: The Citric Acid Cycle93 Questions
Exam 14: Electron Transport and Atp Synthesis95 Questions
Exam 15: Photosynthesis89 Questions
Exam 16: Lipid Metabolism89 Questions
Exam 17: Amino Acid Metabolism84 Questions
Exam 18: Nucleotide Metabolism81 Questions
Exam 19: Nucleic Acids95 Questions
Exam 20: DNA Replication, repair, and Recombination89 Questions
Exam 21: Transcription and RNA Processing91 Questions
Exam 22: Protein Synthesis99 Questions
Select questions type
ATP is classified as an energy-rich compound because
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
D
Enzymes that oxidize NADH can best be measured by the ________ .
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
B
Calculate the equilibrium constant for an oxidation-reduction reaction involving the transfer of 1 mole of electrons with ΔE0'= 0.25 V.(R = 8.315 JK-1mol-1,ℱ= 96.48 kJV-1mol-1,T = 298k)
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
C
Studies of specific enzymes in cells may not give information about cell metabolism since
(Multiple Choice)
4.7/5  (37)
(37)
Calculate △G°'for the reaction shown if the mass action ratio,Q,is 5.3 × 102 M-1 and △G is 46 kJ/mol at a temperature of 298 K.(R = 8.315 J/K-mol)ADP + Pi ⇌ ATP
(Multiple Choice)
4.9/5  (43)
(43)
A chemical reaction with a negative enthalpy change and a negative entropy change ________.
(Multiple Choice)
4.8/5  (39)
(39)
Mutant organisms can be helpful in the study of metabolism because
(Multiple Choice)
5.0/5  (33)
(33)
Both NADH and NADPH are oxidized in cells,but NADH is more often used in specialized pathways to provide hydride ions for reductive reactions in anabolism.
(True/False)
4.8/5  (31)
(31)
For a step in a reaction pathway to serve as a control point it should be ________.
(Multiple Choice)
4.7/5  (38)
(38)
g-Glutamyl phosphate is formed as an intermediate in the conversion of glutamate to glutamine by the enzyme glutamine synthetase because
(Multiple Choice)
4.8/5  (27)
(27)
The free energy for ATP hydrolysis in vivo is actually greater than the standard free energy change of -30 kJ mol-1 because
(Multiple Choice)
4.8/5  (37)
(37)
ATP is thermodynamically suited as a carrier of phosphoryl groups in animal cells because
(Multiple Choice)
4.9/5  (39)
(39)
Which bond in ATP is primarily responsible for its being a high energy molecule? 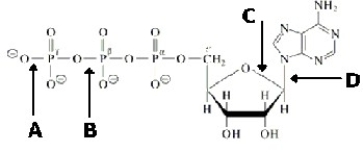
(Multiple Choice)
5.0/5  (26)
(26)
A reaction that best serves as a control point for regulation ________.
(Multiple Choice)
4.8/5  (32)
(32)
The flow of material through a metabolic pathway is called the ________.
(Multiple Choice)
4.7/5  (32)
(32)
Which thermodynamic quantity is used to determine if a reaction in a cell is spontaneous?
(Multiple Choice)
5.0/5  (43)
(43)
Showing 1 - 20 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)