Exam 10: Introduction to Metabolism
Exam 1: Introduction to Biochemistry72 Questions
Exam 2: Water94 Questions
Exam 3: Amino Acids and the Primary Structures of Proteins107 Questions
Exam 4: Proteins: Three-Dimensional Structure and Function116 Questions
Exam 5: Properties of Enzymes91 Questions
Exam 6: Mechanisms of Enzymes88 Questions
Exam 7: Coenzymes and Vitamins93 Questions
Exam 8: Carbohydrates92 Questions
Exam 9: Lipids and Membranes95 Questions
Exam 10: Introduction to Metabolism87 Questions
Exam 11: Glycolysis88 Questions
Exam 12: Gluconeogenesis, the Pentose Phosphate Pathway, and Glycogen Metabolism90 Questions
Exam 13: The Citric Acid Cycle93 Questions
Exam 14: Electron Transport and Atp Synthesis95 Questions
Exam 15: Photosynthesis89 Questions
Exam 16: Lipid Metabolism89 Questions
Exam 17: Amino Acid Metabolism84 Questions
Exam 18: Nucleotide Metabolism81 Questions
Exam 19: Nucleic Acids95 Questions
Exam 20: DNA Replication, repair, and Recombination89 Questions
Exam 21: Transcription and RNA Processing91 Questions
Exam 22: Protein Synthesis99 Questions
Select questions type
A distinct set of metabolic reactions is called a reaction ________.
(Multiple Choice)
4.8/5  (28)
(28)
The ultimate product of complete oxidation of carbohydrates is ________.
(Multiple Choice)
4.8/5  (40)
(40)
Protein folding and lipid-bilayer formation result in decreased entropy for a protein molecule and bilayer components.The decrease in entropy is balanced by a large increase in the entropy of the surrounding ________.
(Multiple Choice)
4.8/5  (31)
(31)
What important high energy functional group is shown in molecule shown? 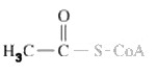
(Multiple Choice)
4.9/5  (48)
(48)
Which is usually the slowest way to regulate a reaction in a metabolic pathway?
(Multiple Choice)
4.8/5  (31)
(31)
What function group leads to ATP being an "energy-rich" compound?
(Multiple Choice)
4.9/5  (39)
(39)
Many dehydrogenases can be assayed by measuring the decrease of absorption of NAD+ or the increase of absorption of NADH.
(True/False)
4.9/5  (39)
(39)
If the standard reduction potential of a certain half-reaction is -0.30 V,which statement below is true for this half-reaction to proceed as a reduction?
(Multiple Choice)
4.9/5  (23)
(23)
Which statement does not explain why many biochemical processes are carried out via multi-step pathways rather than by single-step reactions (or only a few steps)?
(Multiple Choice)
4.8/5  (42)
(42)
The synthesis of acetyl CoA shows that another important role for ATP in cells is to provide its nucleotidyl group for transfer in metabolism.
(True/False)
4.9/5  (28)
(28)
The degradation of which class of biochemicals does not significantly contribute to the release of energy to cells?
(Multiple Choice)
4.8/5  (33)
(33)
The presence of Mg²+ ions decreases the free energy released by the hydrolysis of ATP.
(True/False)
4.7/5  (28)
(28)
Phosphorylation at the expense of ATP is catalyzed by ________.
(Multiple Choice)
4.8/5  (27)
(27)
The percent of genomes involved in intermediary and energy metabolisms in a wide variety of species is about
(Multiple Choice)
4.8/5  (30)
(30)
By linking a reaction that would not ordinarily proceed to the hydrolysis of ATP,the overall coupled reaction may be completed.
(True/False)
4.7/5  (32)
(32)
Metabolic pathways that involve the degradation of large molecules to smaller ones are classified as ________.
(Multiple Choice)
4.8/5  (32)
(32)
Anabolic and catabolic reactions in eukaryotes can occur simultaneously in cells.This is possible because ________.
(Multiple Choice)
4.8/5  (38)
(38)
The process of dissolving table salt,NaCl,in water involves an increase in entropy.
(True/False)
4.7/5  (34)
(34)
Showing 61 - 80 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)