Exam 6: Mechanisms of Enzymes
Exam 1: Introduction to Biochemistry72 Questions
Exam 2: Water94 Questions
Exam 3: Amino Acids and the Primary Structures of Proteins107 Questions
Exam 4: Proteins: Three-Dimensional Structure and Function116 Questions
Exam 5: Properties of Enzymes91 Questions
Exam 6: Mechanisms of Enzymes88 Questions
Exam 7: Coenzymes and Vitamins93 Questions
Exam 8: Carbohydrates92 Questions
Exam 9: Lipids and Membranes95 Questions
Exam 10: Introduction to Metabolism87 Questions
Exam 11: Glycolysis88 Questions
Exam 12: Gluconeogenesis, the Pentose Phosphate Pathway, and Glycogen Metabolism90 Questions
Exam 13: The Citric Acid Cycle93 Questions
Exam 14: Electron Transport and Atp Synthesis95 Questions
Exam 15: Photosynthesis89 Questions
Exam 16: Lipid Metabolism89 Questions
Exam 17: Amino Acid Metabolism84 Questions
Exam 18: Nucleotide Metabolism81 Questions
Exam 19: Nucleic Acids95 Questions
Exam 20: DNA Replication, repair, and Recombination89 Questions
Exam 21: Transcription and RNA Processing91 Questions
Exam 22: Protein Synthesis99 Questions
Select questions type
A reaction that occurs with every collision between reactant molecules is called a(n)________.
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
B
Experiments on the bacterial serine protease subtilisin show that even when all three residues of the catalytic triad are mutated,the catalytic rate of the enzyme is still 3000 times the uncatalyzed reaction rate.Which mode of catalysis is likely responsible for this remaining catalytic activity?
Free
(Multiple Choice)
5.0/5  (37)
(37)
Correct Answer:
C
A nucleophile is an electron poor species.
Free
(True/False)
4.9/5  (37)
(37)
Correct Answer:
False
Aspartate and lysine are in the active site of an enzyme.They are both known to participate directly in catalysis.The pKa's of the residues are found to be 3.2 and 9.6,respectively for aspartate and lysine.The optimum pH for the enzyme is 6.4.Which forms of these two residues will predominate when the enzyme is most active?
(Multiple Choice)
4.7/5  (40)
(40)
The role of ser-195 in chymotrypsin cleavage of a peptide bond is that of a(n)
(Multiple Choice)
4.9/5  (38)
(38)
Trypsin as well as chymotrypsin can activate chymotrypsinogen to chymotrypsin.
(True/False)
4.7/5  (34)
(34)
Histidine is an ideal amino acid at neutral pH values at the active site of many enzymes because
(Multiple Choice)
4.8/5  (34)
(34)
When the concentration of a substrate inside a cell falls below the substrate concentration at half maximal velocity,________.
(Multiple Choice)
4.8/5  (39)
(39)
The graphs below all represent the same chemical reaction,but each employing a different catalyst.Which enzyme uses the most efficient mechanism of catalysis? 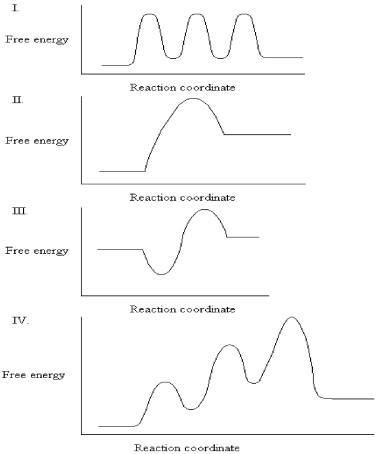
(Multiple Choice)
4.8/5  (38)
(38)
The proximity effect of speeding an enzyme-catalyzed reaction is explained by ________.
(Multiple Choice)
5.0/5  (29)
(29)
X-ray crystallographic examination of the active site of arginine kinase was possible because
(Multiple Choice)
4.8/5  (34)
(34)
Enzymes which have induced fit to the substrate are generally more effective than those in an active form initially.
(True/False)
4.8/5  (32)
(32)
In the reaction below,Y- is ________. Y- + CH₂X → CH₂Y + X-
(Multiple Choice)
4.8/5  (41)
(41)
In the Ser-His-Asp catalytic triad in serine proteases,the serine residue serves as an acid-base catalyst,while the histidine residue serves as a covalent catalyst.
(True/False)
4.9/5  (42)
(42)
In the following chemical reaction which species is the reducing agent?
CH₄ + 2 O2 → CO2 + 2 H₂O
(Multiple Choice)
4.8/5  (33)
(33)
Weak substrate binding is an important feature to help describe enzyme catalysis.
(True/False)
4.9/5  (39)
(39)
Unlike lysozyme,other glycoside hydrolases such as bacterial cellulase,form covalent glycosyl-enzyme intermediates.
(True/False)
4.8/5  (39)
(39)
Replacement of the amino acid ________ at or near an active site of an enzyme is more likely to change enzyme activity than the replacement of ________ at or near the active site.
(Multiple Choice)
4.9/5  (30)
(30)
Showing 1 - 20 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)