Exam 2: The Chemistry of Life
Exam 1: The Nature of Science and the Characteristics of Life80 Questions
Exam 2: The Chemistry of Life93 Questions
Exam 3: Cell Structure and Internal Compartments89 Questions
Exam 4: Cell Membranes, Transport, and Communication89 Questions
Exam 5: Energy, Metabolism, and Enzymes85 Questions
Exam 6: Photo Synthesis and Cellular Respiration89 Questions
Exam 7: Cell Division84 Questions
Exam 8: Cancer and Human Health86 Questions
Exam 9: Patterns of Inheritance86 Questions
Exam 10: Chromosomes and Human Genetics77 Questions
Exam 11: DNA and Genes83 Questions
Exam 12: From Gene to Protein88 Questions
Exam 13: DNA Technology96 Questions
Exam 14: How Evolution Works72 Questions
Exam 15: The Origin of Species76 Questions
Exam 16: The Evolution of Biodiversity102 Questions
Exam 17: Biological Diversity, Bacteria, and Archaea80 Questions
Exam 18: Protista, Plantae, and Fungi83 Questions
Exam 19: Animalia80 Questions
Exam 20: The Biosphere88 Questions
Exam 21: Growth of Populations70 Questions
Exam 22: Animal Behavior82 Questions
Exam 23: Ecological Communities105 Questions
Exam 24: Ecosystems74 Questions
Exam 25: Global Change73 Questions
Select questions type
A monosaccharide is made up of several sugar molecules strung together.
Free
(True/False)
4.9/5  (43)
(43)
Correct Answer:
False
Most lipids contain one or more of the long,hydrophobic hydrocarbon chains known as ________.
Free
(Short Answer)
4.9/5  (30)
(30)
Correct Answer:
fatty acids
In the equation 2 H₂O₂ à 2 H₂O + O₂,the H₂O₂ molecules are the ________ and the H₂O + O₂ molecules are the ________.
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
B
ATP is a universal fuel for living organisms.The energy that ATP molecules deliver in chemical reactions is stored in
(Multiple Choice)
4.9/5  (44)
(44)
Based only on the following illustration,it could be predicted that ice floats on liquid water because 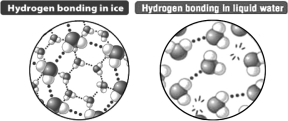
(Multiple Choice)
4.9/5  (30)
(30)
Of the following pH values,which indicates the most basic pH?
(Multiple Choice)
4.7/5  (32)
(32)
Chemical reactions rearrange atoms,but do not create or destroy them.
(True/False)
4.8/5  (37)
(37)
The process of partial hydrogenation turns liquid plant lipids into semisolid lipids by
(Multiple Choice)
4.8/5  (29)
(29)
How many atoms are present in a single molecule of C₈H₁₀N₄O₂?
(Multiple Choice)
4.9/5  (33)
(33)
A type of organic compound that plays a role in both heredity and in energy delivery in cells is a ________.
(Short Answer)
4.8/5  (42)
(42)
The following figure shows two hydrogen atoms.  How many covalent bonds will form between these two atoms?
How many covalent bonds will form between these two atoms?
(Multiple Choice)
4.9/5  (28)
(28)
Showing 1 - 20 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)