Exam 2: Water: The Solvent for Biochemical Reactions
Exam 1: Biochemistry and the Organization of Cells69 Questions
Exam 2: Water: The Solvent for Biochemical Reactions79 Questions
Exam 3: Amino Acids and Peptides77 Questions
Exam 4: The Three-Dimensional Structure of Proteins77 Questions
Exam 5: Protein Purification and Characterization Techniques58 Questions
Exam 6: The Behavior of Proteins: Enzymes78 Questions
Exam 7: The Behavior of Proteins: Enzymes, Mechanisms, and Control77 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes90 Questions
Exam 9: Nucleic Acids: How Structure Conveys Information60 Questions
Exam 10: Biosynthesis of Nucleic Acids: Replication79 Questions
Exam 11: Transcription of the Genetic Code: Biosynthesis of Rna93 Questions
Exam 12: Protein Synthesis: Translation of the Genetic Message80 Questions
Exam 13: Nucleic Acid Biotechnology Techniques89 Questions
Exam 14: Viruses, Cancer, and Immunology35 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism56 Questions
Exam 16: Carbohydrates88 Questions
Exam 17: Glycolysis63 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism79 Questions
Exam 19: The Citric Acid Cycle77 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation70 Questions
Exam 21: Lipid Metabolism86 Questions
Exam 22: Photosynthesis74 Questions
Exam 23: The Metabolism of Nitrogen78 Questions
Exam 24: Integration of Metabolism: Cellular Signaling60 Questions
Select questions type
Which of the following classes of compounds is hydrophobic?
(Multiple Choice)
4.8/5  (31)
(31)
What is the pH of an acetic acid solution where the concentration of acetic acid is 2 mM and the concentration of sodium acetate is 20 mM.The pKa of acetic acid is 4.76.
(Multiple Choice)
4.8/5  (27)
(27)
Exhibit 2A 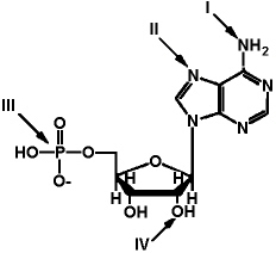 The structure of ATP with various groups labeled.
Group III is the entire phosphate group.
Refer to Exhibit 2A.Which of the functional groups cannot function as a hydrogen donor to water?
The structure of ATP with various groups labeled.
Group III is the entire phosphate group.
Refer to Exhibit 2A.Which of the functional groups cannot function as a hydrogen donor to water?
(Multiple Choice)
4.8/5  (31)
(31)
Which will dissociate most in water,a weak acid or a strong acid?
(Multiple Choice)
4.7/5  (41)
(41)
How does the strength of hydrogen bonds compare with covalent bonds?
(Multiple Choice)
4.8/5  (40)
(40)
How do hydrogen bonds tend to affect the melting and boiling points of substances?
(Multiple Choice)
4.9/5  (37)
(37)
True hydrogen bonds can NOT form between hydrogen and this element:
(Multiple Choice)
4.9/5  (41)
(41)
The dissociation constant for an acid is 1 × 10-6.What is its pKa?
(Multiple Choice)
4.8/5  (41)
(41)
Which substance would be the best buffer at pH 8 if it had to be able to buffer against either acid or base?
(Multiple Choice)
4.8/5  (40)
(40)
The non-covalent interaction below associated with the strongest force in aqueous solution is
(Multiple Choice)
4.7/5  (36)
(36)
Exhibit 2B Contains information on the pK's of some common buffers.
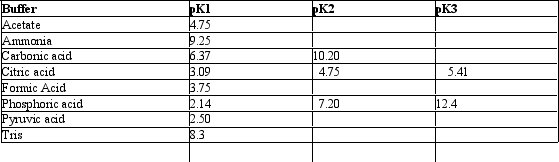 Refer to Exhibit 2B.A phosphate buffer would work well at this pH:
Refer to Exhibit 2B.A phosphate buffer would work well at this pH:
(Multiple Choice)
4.9/5  (28)
(28)
Consider a reaction that produces a significant amount of hydrogen ion and is to be carried out a pH 7.Only two acids are available for making the buffer solution.The pKa values for acids A and B are 6.3 and 7.3,respectively.Which acid would serve as the optimum buffer for this reaction? Or would carrying out the reaction in water simply serve as well?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 21 - 40 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)