Exam 2: Water: The Solvent for Biochemical Reactions
Exam 1: Biochemistry and the Organization of Cells69 Questions
Exam 2: Water: The Solvent for Biochemical Reactions79 Questions
Exam 3: Amino Acids and Peptides77 Questions
Exam 4: The Three-Dimensional Structure of Proteins77 Questions
Exam 5: Protein Purification and Characterization Techniques58 Questions
Exam 6: The Behavior of Proteins: Enzymes78 Questions
Exam 7: The Behavior of Proteins: Enzymes, Mechanisms, and Control77 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes90 Questions
Exam 9: Nucleic Acids: How Structure Conveys Information60 Questions
Exam 10: Biosynthesis of Nucleic Acids: Replication79 Questions
Exam 11: Transcription of the Genetic Code: Biosynthesis of Rna93 Questions
Exam 12: Protein Synthesis: Translation of the Genetic Message80 Questions
Exam 13: Nucleic Acid Biotechnology Techniques89 Questions
Exam 14: Viruses, Cancer, and Immunology35 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism56 Questions
Exam 16: Carbohydrates88 Questions
Exam 17: Glycolysis63 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism79 Questions
Exam 19: The Citric Acid Cycle77 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation70 Questions
Exam 21: Lipid Metabolism86 Questions
Exam 22: Photosynthesis74 Questions
Exam 23: The Metabolism of Nitrogen78 Questions
Exam 24: Integration of Metabolism: Cellular Signaling60 Questions
Select questions type
Using the Henderson-Hasselbalch equation,calculate the pH of an ammonia buffer when the NH3:NH4+ ratio is 0.4 moles:0.6 moles.(pK = 9.75)
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following molecules will not form hydrogen bonds?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following compounds is most likely to form a micelle?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is a correct listing of electronegativity values,from low to high?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following characteristics makes for a good hydrogen bond acceptor?
(Multiple Choice)
4.8/5  (36)
(36)
If the pH of 1 liter of a 1.0 M carbonate buffer is 7.0,what is the molar ratio of H2CO3 to HCO3-? (pK = 6.37)
(Multiple Choice)
4.9/5  (30)
(30)
Ionic compounds and polar covalent compounds tend to dissolve in water because of
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following characteristics makes for a good hydrogen bond donor?
(Multiple Choice)
4.8/5  (39)
(39)
Many of the properties of water can be accounted for by the fact that
(Multiple Choice)
4.9/5  (41)
(41)
A solution at pH 7 contains a weak acid,HA.The pKa of the acid is 6.5.What is the ratio of [A-]:[HA]?
(Multiple Choice)
4.8/5  (36)
(36)
Exhibit 2A 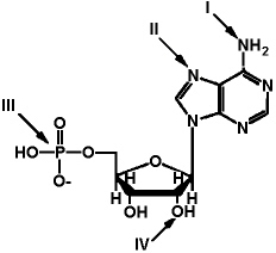 The structure of ATP with various groups labeled.
Group III is the entire phosphate group.
Refer to Exhibit 2A.Which of the functional groups is the most electrophilic?
The structure of ATP with various groups labeled.
Group III is the entire phosphate group.
Refer to Exhibit 2A.Which of the functional groups is the most electrophilic?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following acids would serve as a good buffer for a reaction at pH = 8.0? 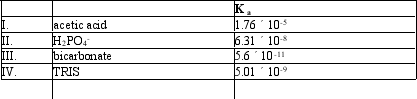
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following elements has the highest electronegativity?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 61 - 79 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)