Exam 9: Introduction to Solutions and Aqueous Reactions
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
Balance the chemical equation given below,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 8.00 g of oxygen at 25°C? The density of nitrogen monoxide at 25°C is 1.23 g/L.
________ NH3(g)+ ________ O2(g)→________ NO(g)+ ________ H2O(l)
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following is a strong electrolyte in solution?
(Multiple Choice)
4.9/5  (44)
(44)
How many moles of 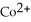 are present in 0.550 L of a 0.550 M solution of Co
are present in 0.550 L of a 0.550 M solution of Co 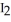 ?
?
(Short Answer)
4.8/5  (40)
(40)
94.20 mL of 0.800 M potassium hydroxide reacts with 125.0 mL of a solution containing oxalic acid,which is a diprotic species.What is the concentration of the acid solution?
(Multiple Choice)
4.9/5  (21)
(21)
Pure acetic acid ( 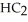
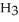
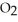 )is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.
)is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.
(Multiple Choice)
4.8/5  (42)
(42)
Determine the molarity of a solution formed by dissolving 468 mg of MgI2 in enough water to yield 50.0 mL of solution.
(Multiple Choice)
4.9/5  (32)
(32)
Describe the difference between complete ionic and net ionic equations.
(Essay)
4.8/5  (34)
(34)
How many liters of a 0.0550 M Li F solution contain 0.163 moles of Li F?
(Multiple Choice)
4.7/5  (30)
(30)
How would the concentration change if a 1.0 L flask of 1.0 M NaCl were left uncapped on a laboratory bench for several days.Why?
(Essay)
4.8/5  (37)
(37)
The titration of 25.0 mL of an unknown concentration H2SO4 solution requires 83.6 mL of 0.12 M LiOH solution.What is the concentration of the H2SO4 solution (in M)?
(Multiple Choice)
4.8/5  (35)
(35)
How many aluminum ions are present in 65.5 mL of 0.210 M Al I3 solution?
(Multiple Choice)
4.9/5  (43)
(43)
Based on the balanced chemical equation shown below,determine the molarity of a solution containing Fe2+(aq),if 40.00 mL of the Fe2+(aq)solution is required to completely react with 30.00 mL of a 0.125 M potassium bromate,KBrO3(aq),solution.The chemical equation for the reaction is 6 Fe2+(aq)+ BrO3-(aq)+ 6 H+(aq)→ 6 Fe3+(aq)+ Br-(aq)+ 3 H2O(l).
(Multiple Choice)
4.8/5  (37)
(37)
Identify the oxidation state of Ca in Ca(s). Ca(s)+ 2H F(aq)→ Ca F2(aq)+ H2(g)
(Multiple Choice)
4.9/5  (34)
(34)
Showing 121 - 140 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)