Exam 9: Introduction to Solutions and Aqueous Reactions
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
Give the net ionic equation for the reaction (if any)that occurs when aqueous solutions of Na2CO3 and HCl are mixed.
(Multiple Choice)
4.9/5  (34)
(34)
Balance the chemical equation given below,and determine the number of milliliters of 0.00300 M phosphoric acid required to neutralize 40.00 mL of 0.00150 M calcium hydroxide. ________ Ca(OH)2(aq)+ ________ H3PO4(aq)→ ________ Ca3(PO4)2(aq)+ ________ H2O(l)
(Multiple Choice)
4.8/5  (38)
(38)
Determine the concentration of a solution prepared by diluting 20.0 mL of a 0.200 M KCl to 250.0 mL.
(Multiple Choice)
4.8/5  (51)
(51)
Explain the difference between a strong and weak electrolyte.Give an example of each.
(Essay)
4.9/5  (31)
(31)
A solution is prepared by adding 1.50 g of solid NaCl to 50.0 mL of 0.100 M 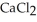 .What is the molarity of chloride ion in the final solution? Assume that the volume of the final solution is 50.0 mL.
.What is the molarity of chloride ion in the final solution? Assume that the volume of the final solution is 50.0 mL.
(Multiple Choice)
4.9/5  (39)
(39)
Which one of the following compounds is insoluble in water?
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following pairs of aqueous solutions will form a precipitate when mixed?
(Multiple Choice)
5.0/5  (35)
(35)
What reagent could be used to separate Br- from N O3- when added to an aqueous solution containing both?
(Multiple Choice)
4.7/5  (44)
(44)
Which of the following pairs of aqueous solutions will form a precipitate when mixed?
(Multiple Choice)
4.9/5  (45)
(45)
What element is undergoing oxidation (if any)in the following reaction?
CH4(g)+ 2 O2(g)→ CO2(g)+ 2 H2O(g)
(Multiple Choice)
4.8/5  (25)
(25)
What is the concentration of nitrate ions in a 0.125 M Ba(NO3)2 solution?
(Multiple Choice)
4.8/5  (41)
(41)
How many milliliters of a 9.0 M H2SO4 solution are needed to make 0.45 L of a 3.5 M solution?
(Multiple Choice)
5.0/5  (33)
(33)
Which of the following compounds is NOT an Arrhenius acid?
(Multiple Choice)
4.8/5  (44)
(44)
What volume of 1.46 M CrBr2 solution is required to react with 50.0 mL of 0.210 M Na2CO3 solution?
CrBr2(aq)+ Na2CO3(aq)→ CrCO3(s)+ 2NaBr(aq)
(Multiple Choice)
4.7/5  (42)
(42)
Showing 161 - 177 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)