Exam 9: Introduction to Solutions and Aqueous Reactions
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
What is the molarity of a NaOH solution if 39.1 mL of a 0.112 M 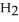
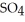 solution is required to neutralize a 25.0-mL sample of the NaOH solution?
solution is required to neutralize a 25.0-mL sample of the NaOH solution?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following pairs of aqueous solutions will form a precipitate when mixed?
(Multiple Choice)
4.9/5  (35)
(35)
Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride: 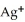 (aq)+
(aq)+ 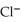 (aq)→ AgCl(s) Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.175 M
(aq)→ AgCl(s) Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.175 M 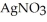 solution to completely precipitate the silver?
solution to completely precipitate the silver?
(Multiple Choice)
4.8/5  (48)
(48)
In an acid-base neutralization reaction 23.74 mL of 0.500 M potassium hydroxide reacts with 50.00 mL of sulfuric acid solution.What is the concentration of the H2SO4 solution?
(Multiple Choice)
4.9/5  (43)
(43)
If 100.mL of 0.200 M Na2SO4 is added to 200.mL of 0.300 M NaCl,what is the concentration of Na+ ions in the final solution? Assume that the volumes are additive.
(Multiple Choice)
4.8/5  (40)
(40)
According to the following reaction,what volume of 0.244 M KCl solution is required to react exactly with 50.0 mL of 0.210 M Pb(NO3)2 solution?
2 KCl(aq)+ Pb(NO3)2(aq)→ PbCl2(s)+ 2 KNO3(aq)
(Multiple Choice)
4.8/5  (35)
(35)
What element is undergoing oxidation (if any)in the following reaction?
2 MnO4-(aq)+ 3 NO2-(aq)+ H2O(l)→ 2 MnO2(s)+ 3 NO3-(aq)+ 2 OH-(aq)
(Multiple Choice)
4.9/5  (40)
(40)
What mass (in g)of AgCl is formed from the reaction of 75.0 mL of a 0.078 M AgC2H3O2 solution with 55.0 mL of 0.109 M MgCl2 solution?
2 AgC2H3O2(aq)+ MgCl2(aq)→ 2 AgCl(s)+ Mg(C2H3O2)2(aq)
(Multiple Choice)
4.9/5  (38)
(38)
How many grams of 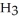 P
P 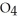 are in 325 mL of a 5.50 M solution of
are in 325 mL of a 5.50 M solution of 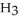 P
P 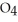 ?
?
(Short Answer)
4.9/5  (39)
(39)
What volume of 0.305 M AgNO3 is required to react exactly with 155.0 mL of 0.274 M Na2SO4 solution? Hint: You will want to write a balanced reaction.
(Multiple Choice)
4.9/5  (30)
(30)
Based on the balanced chemical equation shown below,what volume of 0.250 M K2S2O3(aq),is needed to completely react with 12.44 mL of 0.125 M KI3(aq),according to the following chemical equation. 2 S2O32-(aq)+ I3-(aq)→ S4O62-(aq)+ 3 I-(aq)
(Multiple Choice)
4.9/5  (39)
(39)
How many of the following compounds are soluble in water?
Cu(OH)2 LiNO3 NH4I Na2S
(Multiple Choice)
4.9/5  (32)
(32)
How many H+ ions can the acid,H2SeO4 ,donate per molecule?
(Multiple Choice)
4.9/5  (39)
(39)
Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 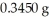 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq),solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq)+ Sn2+(aq)→ 2 Fe2+(aq)+ Sn4+(aq).
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq),solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq)+ Sn2+(aq)→ 2 Fe2+(aq)+ Sn4+(aq).
(Multiple Choice)
4.7/5  (32)
(32)
Showing 141 - 160 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)