Exam 16: Temperature and Heat
Exam 1: Introduction to Physics98 Questions
Exam 2: One-Dimensional Kinematics138 Questions
Exam 3: Vectors in Physics69 Questions
Exam 4: Two-Dimensional Kinematics51 Questions
Exam 5: Newtons Laws of Motion54 Questions
Exam 6: Applications of Newtons Laws104 Questions
Exam 7: Work and Kinetic Energy55 Questions
Exam 8: Potential Energy and Conservation of Energy62 Questions
Exam 9: Linear Momentum and Collisions114 Questions
Exam 10: Rotational Kinematics and Energy60 Questions
Exam 11: Rotational Dynamics and Static Equilibrium69 Questions
Exam 12: Gravity53 Questions
Exam 13: Oscillations About Equilibrium79 Questions
Exam 14: Waves and Sound142 Questions
Exam 15: Fluids103 Questions
Exam 16: Temperature and Heat110 Questions
Exam 17: Phases and Phase Changes93 Questions
Exam 18: The Laws of Thermodynamics90 Questions
Exam 19: Electric Charges, Forces, and Fields75 Questions
Exam 20: Electric Potential and Electric124 Questions
Exam 21: Electric Current and Direct-Current Circuits228 Questions
Exam 22: Magnetism147 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction98 Questions
Exam 24: Alternating-Current Circuits72 Questions
Exam 25: Electromagnetic Waves63 Questions
Exam 26: Geometrical Optics133 Questions
Exam 27: Optical Instruments103 Questions
Exam 28: Physical Optics: Interference and Diffraction119 Questions
Exam 29: Relativity98 Questions
Exam 30: Quantum Physics88 Questions
Exam 31: Atomic Physics97 Questions
Exam 32: Nuclear Physics and Nuclear Radiation137 Questions
Select questions type
A person running in place on an exercise machine for 10 min uses up 17 kcal (food calories).Another person exercises by repeatedly lifting two 2.5-kg weights a distance of 50 cm.How many repetitions of this exercise are equivalent to 10 minutes of running in place? Assume that the person uses negligible energy in letting down the weights after each lift.(1 cal = 4.186 J)
(Multiple Choice)
4.8/5  (38)
(38)
In grinding a steel knife,the metal can get as hot as 400°C.If the blade has a mass of 80 g,what is the minimum amount of water needed at 20°C if the water is to remain liquid and not rise above 100°C when the hot blade is quenched in it? The specific heat of the steel is 0.11 cal/g ∙ °C and the specific heat of water is 1.0 cal/g ∙ °C.
(Multiple Choice)
4.9/5  (38)
(38)
Two identical objects are placed in a room with a temperature of 20°C.Object A has a temperature of 50°C,while object B has a temperature of 90°C.What is the ratio of the net power emitted by object B to the power emitted by object A?
(Multiple Choice)
4.9/5  (44)
(44)
The coefficient of linear expansion of steel is 12 × 10-6 K-1.What is the change in length of a 25-m steel bridge span when it undergoes a temperature change of 40 K from winter to summer?
(Multiple Choice)
4.9/5  (43)
(43)
A person consumes a snack containing 14 food calories (14 kcal).What is the power this food produces if it is to be "burned off" due to exercise in 6 hours? (1 cal = 4.186 J)
(Multiple Choice)
4.9/5  (38)
(38)
A heat conducting rod,1.60 m long and wrapped in insulation,is made of an aluminum section that is 0.90 m long and a copper section that is 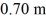 Long.Both sections have a cross-sectional area of
Long.Both sections have a cross-sectional area of 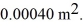 The aluminum end and the copper end are maintained at temperatures of
The aluminum end and the copper end are maintained at temperatures of 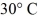 And
And  Respectively.The thermal conductivities of aluminum and copper are 205 W/m ∙ K (aluminum)and 385 W/m ∙ K (copper).At what rate is heat conducted in the rod under steady state conditions?
Respectively.The thermal conductivities of aluminum and copper are 205 W/m ∙ K (aluminum)and 385 W/m ∙ K (copper).At what rate is heat conducted in the rod under steady state conditions?
(Multiple Choice)
4.9/5  (31)
(31)
Two metal rods,one silver and the other gold,are attached to each other end-to-end.The free end of the silver rod is immersed in a steam chamber at 100°C,and the free end of the gold rod in an ice water bath at 0°C.The rods are both 5.0 cm long and have a square cross-section that is 2.0 cm on a side.No heat is exchanged between the rods and their surroundings,except at the ends.What is the temperature at the point where the two rods are in contact with one another? The thermal conductivity of silver is 417 W/m ∙ K,and that of gold is 291 W/m ∙ K.
(Multiple Choice)
4.8/5  (43)
(43)
How much power does a sphere with a radius of 10 cm radiate into empty space if is has an emissivity of 1.0 and is kept at a temperature of 400 K? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.8/5  (39)
(39)
The coefficient of linear expansion of aluminum is 24 × 10-6 K-1 and the coefficient of volume expansion of olive oil is 0.68 × 10-3 K-1.A novice cook,in preparation of some pesto,fills a 1.00-L aluminum pot to the brim and heats the oil and the pot from an initial temperature of 15°C to 190°C.To his consternation some olive oil spills over the top.How much?
(Multiple Choice)
4.9/5  (46)
(46)
A large vat contains 1.000 L of water at 20°C.What volume will this water occupy when it is heated up to 80°C? Water has a volume expansion coefficient of 210 × 10-6 K-1.
(Multiple Choice)
4.8/5  (38)
(38)
An architect is interested in estimating the rate of heat loss,ΔQ/Δt,through a sheet of insulating material as a function of the thickness of the sheet.Assuming fixed temperatures on the two faces of the sheet and steady state heat flow,which one of the graphs shown in the figure best represents the rate of heat transfer as a function of the thickness of the insulating sheet? 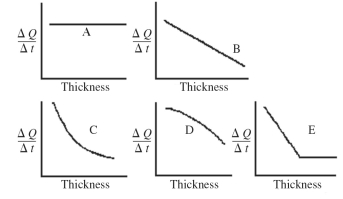
(Multiple Choice)
4.8/5  (30)
(30)
A camper is about to drink his morning coffee.He pours 400 grams of coffee,initially at 75°C into a 250-g aluminum cup,initially at 16°C.What is the equilibrium temperature of the coffee-cup system,assuming no heat is lost to the surroundings? The specific heat of aluminum is 900 J/kg ∙ K,and the specific heat of coffee is essentially the same as that of water,which is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (38)
(38)
If the absolute temperature of an object is tripled,the thermal power radiated by this object (assuming that its emissivity and size are not affected by the temperature change)will
(Multiple Choice)
5.0/5  (42)
(42)
An aluminum rod 17.400 cm long at 20°C is heated to 100°C.What is its new length? Aluminum has a linear expansion coefficient of 25 × 10-6 K-1.
(Multiple Choice)
4.9/5  (35)
(35)
A machine part consists of 0.10 kg of iron (of specific heat 470 J/kg ∙ K )and 0.16 kg of copper (of specific heat 390 J/kg ∙ K).How much heat must be added to the gear to raise its temperature from 18°C to 53°C?
(Multiple Choice)
4.9/5  (25)
(25)
How much heat is required to raise the temperature of a 225-g lead ball from 15.0°C to 25.0°C? The specific heat of lead is 128 J/kg ∙ K.
(Multiple Choice)
4.8/5  (34)
(34)
A 90-g aluminum calorimeter contains 390 g of water at an equilibrium temperature of 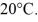 A
A  Piece of metal,initially at
Piece of metal,initially at 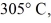 Is added to the calorimeter.The final temperature at equilibrium is 32° C.Assume there is no external heat exchange.The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum)and 4190 J/kg ∙ K (water).What is the specific heat capacity of the 160-g piece of metal?
Is added to the calorimeter.The final temperature at equilibrium is 32° C.Assume there is no external heat exchange.The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum)and 4190 J/kg ∙ K (water).What is the specific heat capacity of the 160-g piece of metal?
(Multiple Choice)
4.8/5  (39)
(39)
A jogger is running outdoors on a cold day when the temperature is -20.0°C.She is breathing at the rate of 25 breaths per minute,and each time she breathes in she inhales 0.00450 m3 of air.How much heat does she lose from breathing during 20.0 minutes of jogging if the air in her lungs is heated to her body temperature of 37.0°C before it is exhaled? The specific heat of air is 1020 J/kg ∙ K and the density of air under typical conditions is 1.29 kg/m3.
(Multiple Choice)
4.9/5  (32)
(32)
A person is walking outdoors on a cold day when the temperature is -20°C.He is breathing at the rate of 16 breaths per minute,and each time he breathes in he inhales 0.0050 m3 of air.At what rate does he lose heat from breathing if the air in his lungs is heated to body temperature (37°C)before it is exhaled? The specific heat of air is 1020 J/kg ∙ K and the density of air is 1.29 kg/m3.
(Multiple Choice)
4.8/5  (37)
(37)
Showing 21 - 40 of 110
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)