Exam 16: Temperature and Heat
Exam 1: Introduction to Physics98 Questions
Exam 2: One-Dimensional Kinematics138 Questions
Exam 3: Vectors in Physics69 Questions
Exam 4: Two-Dimensional Kinematics51 Questions
Exam 5: Newtons Laws of Motion54 Questions
Exam 6: Applications of Newtons Laws104 Questions
Exam 7: Work and Kinetic Energy55 Questions
Exam 8: Potential Energy and Conservation of Energy62 Questions
Exam 9: Linear Momentum and Collisions114 Questions
Exam 10: Rotational Kinematics and Energy60 Questions
Exam 11: Rotational Dynamics and Static Equilibrium69 Questions
Exam 12: Gravity53 Questions
Exam 13: Oscillations About Equilibrium79 Questions
Exam 14: Waves and Sound142 Questions
Exam 15: Fluids103 Questions
Exam 16: Temperature and Heat110 Questions
Exam 17: Phases and Phase Changes93 Questions
Exam 18: The Laws of Thermodynamics90 Questions
Exam 19: Electric Charges, Forces, and Fields75 Questions
Exam 20: Electric Potential and Electric124 Questions
Exam 21: Electric Current and Direct-Current Circuits228 Questions
Exam 22: Magnetism147 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction98 Questions
Exam 24: Alternating-Current Circuits72 Questions
Exam 25: Electromagnetic Waves63 Questions
Exam 26: Geometrical Optics133 Questions
Exam 27: Optical Instruments103 Questions
Exam 28: Physical Optics: Interference and Diffraction119 Questions
Exam 29: Relativity98 Questions
Exam 30: Quantum Physics88 Questions
Exam 31: Atomic Physics97 Questions
Exam 32: Nuclear Physics and Nuclear Radiation137 Questions
Select questions type
A 4.0-kg aluminum block is originally at 10°C.If 160 kJ of heat is added to the block,what is its final temperature? The specific heat capacity of aluminum is 910 J/kg ∙ K.
(Multiple Choice)
4.8/5  (36)
(36)
A glass tea kettle containing 500 g of water is on the stove.The portion of the tea kettle that is in contact with the heating element has an area of 0.090 m2 and is 1.5 mm thick.At a certain moment,the temperature of the water is 75°C,and it is rising at the rate of 3°C per minute.What is the temperature of the outside surface of the bottom of the tea kettle? Neglect the heat capacity of the kettle,and assume that the inner surface of the kettle is at the same temperature as the water inside.The thermal conductivity of glass is 0.840 W/m ∙ K and the specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (44)
(44)
A 200-L electric water heater uses 2.0 kW.Assuming no heat loss,how many hours would it take to heat the water in this tank from 23°C to 75°C? The specific heat of water is 4186 J/kg ∙ K and its density is 1000 kg/m3.
(Multiple Choice)
4.9/5  (40)
(40)
What is the net power that a person with surface area of 1.20 m2 radiates if his emissivity is 0.895,his skin temperature is 27°C,and he is in a room that is at a temperature of 17°C? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.9/5  (38)
(38)
A quantity of mercury occupies 400.0 cm3 at 0°C.What volume will it occupy when heated to 50°C? Mercury has a volume expansion coefficient of 180 × 10-6 K-1.
(Multiple Choice)
4.9/5  (35)
(35)
The radius of a star is 6.95 × 108 m,and its rate of radiation has been measured to be 5.32 × 1026 W.Assuming that it is a perfect emitter,what is the temperature of the surface of this star? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.9/5  (42)
(42)
Two metal rods,one silver and the other copper,are both immersed at one end in a steam chamber at a temperature of 100°C.The other end of each one is in an ice water bath at 0°C.The rods are 5.0 cm long and have a square cross-section that is 2.0 cm on a side.No heat is exchanged between the rods and the surroundings,except at the ends.How much total heat flows through the two rods each minute? The thermal conductivity of silver is 417 W/m ∙ K,and that of copper is 395 W/m ∙ K.
(Multiple Choice)
4.9/5  (46)
(46)
The cylindrical filament in a light bulb has a diameter of 0.050 mm,an emissivity of 1.0,and a temperature of 3000°C.How long should the filament be in order to radiate 60 W of power? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.8/5  (28)
(28)
The water flowing over Niagara Falls drops a distance of 50 m.If all the gravitational potential energy is converted to thermal energy,by what temperature does the water rise? The specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.7/5  (34)
(34)
The coefficient of linear expansion of copper is 17 × 10-6 K-1.A block of copper 30 cm wide,45 cm long,and 10 cm thick is heated from 0°C to 100°C What is the change in the volume of the block?
(Multiple Choice)
4.9/5  (32)
(32)
Object 1 has three times the specific heat capacity and four times the mass of Object 2.The two objects are heated from the same initial temperature,T0,to the same final temperature Tf.During this process,if Object 1 absorbs heat Q,the amount of heat absorbed by Object 2 will be
(Multiple Choice)
4.8/5  (34)
(34)
The coefficient of volume expansion of a certain olive oil is 0.68 × 10-3 K-1.A 1.0-L glass beaker is filled to the brim with olive oil at room temperature.The beaker is placed on a range and the temperature of the oil and beaker increases by 25°C.As a result,0.0167 L of olive oil spills over the top of the beaker.Which of the following values is closest to the coefficient of linear expansion of the glass from which the beaker is made?
(Multiple Choice)
4.9/5  (31)
(31)
A 5.00-g lead BB moving at 44.0 m/s penetrates a wood block and comes to rest inside the block.If half of its kinetic energy is absorbed by the BB,what is the change in the temperature of the BB? The specific heat of lead is 128 J/kg ∙ K.
(Multiple Choice)
4.8/5  (41)
(41)
A lab student drops a 400.0-g piece of metal at 120.0°C into a cup containing 450.0 g of water at 15.0°C.After waiting for a few minutes,the student measures that the final temperature of the system is 40.0°C.What is the specific heat of the metal,assuming that no significant heat is exchanged with the surroundings or the cup? The specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (40)
(40)
Two metal spheres are made of the same material and have the same diameter,but one is solid and the other is hollow.If their temperature is increased by the same amount,
(Multiple Choice)
4.9/5  (43)
(43)
It is necessary to determine the specific heat of an unknown object.The mass of the object is 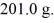 It is determined experimentally that it takes
It is determined experimentally that it takes 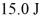 To raise the temperature
To raise the temperature 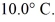 What is the specific heat of the object?
What is the specific heat of the object?
(Multiple Choice)
4.9/5  (44)
(44)
An aluminum electric tea kettle with a mass of 500 g is heated with a 500-W heating coil.How long will it take to heat up 1.0 kg of water from 18°C to 98°C in the tea kettle? The specific heat of aluminum is 900 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (40)
(40)
A window glass that is 0.5 cm thick has dimensions of 3 m by 1.5 m.The thermal conductivity of this glass is 0.8 W/m ∙ K.If the outside surface of the glass is at -10°C and the inside surface is at 20°C,how much heat flows through the window in every hour?
(Multiple Choice)
4.9/5  (34)
(34)
If you wanted to know how much the temperature of a particular piece of material would rise when a known amount of heat was added to it,which of the following quantities would be most helpful to know?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 41 - 60 of 110
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)