Exam 20: Transition Elements and Coordination Chemistry
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
How many unpaired electrons will Co have in the complex [CoCl4]2-?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following elements is an inner transition metal?
(Multiple Choice)
4.8/5  (34)
(34)
Which pair of isomers illustrates the concept of ionization isomers?
(Multiple Choice)
4.8/5  (31)
(31)
The small sizes of third transition series atoms is referred to as the ________ contraction.
(Short Answer)
4.8/5  (31)
(31)
What is the oxidation state of the Co atom in [Co(NH3)5Cl](NO3)2?
(Multiple Choice)
4.9/5  (37)
(37)
What is the ground-state electron configuration for Cr in Cr2O72-?
(Multiple Choice)
4.8/5  (44)
(44)
What is the expected order for increasing octahedral (Δ0)crystal field splitting for the ligands I-,F-,H2O,NH3,en,CO?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following complexes has five unpaired electrons?
(Multiple Choice)
4.8/5  (42)
(42)
For transition elements,which of the following occurs as the effective nuclear charge increases?
(Multiple Choice)
4.8/5  (36)
(36)
What statement is inconsistent with the chemistry of copper?
(Multiple Choice)
4.8/5  (31)
(31)
In the two half-reactions shown below,which chromium species is the strongest oxidizing agent?
Cr2O72-(aq)+ 14 H+(aq)+ 6 e- → 2 Cr3+(aq)+ 7 H2O(l)E° = + 1.33 V
CrO42-(aq)+ 4 H2O(l)+ 6 e- → Cr(OH)3(s)+ 5 OH-(aq)E° = - 0.13 V
(Multiple Choice)
4.9/5  (41)
(41)
What is the ground-state electron configuration for the element cobalt (Z = 27)?
(Multiple Choice)
4.9/5  (42)
(42)
The first transition series metals are shown below. 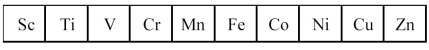 -Which has the most negative standard oxidation potential?
-Which has the most negative standard oxidation potential?
(Multiple Choice)
4.9/5  (43)
(43)
What type of hybrid orbitals are used by the Ti atom to form chemical bonds in the complex ion [Ti(H2O)6]3+?
(Multiple Choice)
4.9/5  (27)
(27)
Which element is most likely to have an anomalous electron configuration?
(Multiple Choice)
4.9/5  (34)
(34)
The number of unpaired electrons in tetrahedral NiBr42- is ________.
(Short Answer)
4.9/5  (41)
(41)
Showing 81 - 100 of 185
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)