Exam 20: Transition Elements and Coordination Chemistry
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
What statement is most inconsistent with the chemistry of transition elements?
(Multiple Choice)
4.9/5  (35)
(35)
The first transition series metals are shown below. 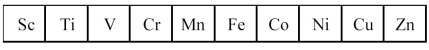 -Which metal exhibits the highest oxidation state in its compounds?
-Which metal exhibits the highest oxidation state in its compounds?
(Multiple Choice)
4.8/5  (35)
(35)
Which is not a characteristic reaction of chromium metal or chromium(II)ion?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following species has the electron configuration [Ar]3d6?
(Multiple Choice)
4.8/5  (42)
(42)
Which one of the following complexes is considered to be tetrahedral?
(Multiple Choice)
4.8/5  (38)
(38)
The first transition series metals are shown below.  -Based on the variation in Zeff,which M2+ ion should be the strongest reducing agent?
-Based on the variation in Zeff,which M2+ ion should be the strongest reducing agent?
(Multiple Choice)
4.8/5  (35)
(35)
What is the highest possible oxidation state for manganese?
(Multiple Choice)
4.9/5  (32)
(32)
Which one of the following compounds is not consistent with the terminology of a complex?
(Multiple Choice)
5.0/5  (29)
(29)
What is the oxidation state of the Cr atom in [Ni(en)3]3[Cr(CN)6]2?
(Multiple Choice)
4.8/5  (38)
(38)
The second transition series metals are shown below. 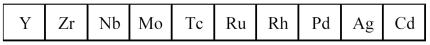 -Which has the highest melting point?
-Which has the highest melting point?
(Multiple Choice)
4.8/5  (29)
(29)
![-Which element indicated on the above periodic table has the electron configuration [Kr] 4d<sup>5</sup> 5s<sup>2</sup>?](https://storage.examlex.com/TB4939/11ea7a38_c9a7_8e4f_aa4c_ff5f6fa7120c_TB4939_00_TB4939_00_TB4939_00_TB4939_00_TB4939_00_TB4939_00.jpg) -Which element indicated on the above periodic table has the electron configuration [Kr] 4d5 5s2?
-Which element indicated on the above periodic table has the electron configuration [Kr] 4d5 5s2?
(Multiple Choice)
4.9/5  (44)
(44)
The third transition series metals are shown below. 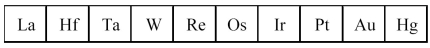 -Which has the smallest atomic radius?
-Which has the smallest atomic radius?
(Multiple Choice)
4.8/5  (32)
(32)
The compounds [Cr(H2O)6]Cl3 and [CrCl3(H2O)3] ∙ 3H2O are examples of ________.
(Multiple Choice)
4.7/5  (37)
(37)
The second transition series metals are shown below.  -Which has the lowest density?
-Which has the lowest density?
(Multiple Choice)
4.8/5  (34)
(34)
Consider the following isomers of [Co(NH3)4Br2]+.The black sphere represents Co,gray spheres represent NH3,and unshaded spheres represent Br. ![Consider the following isomers of [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup>.The black sphere represents Co,gray spheres represent NH<sub>3</sub>,and unshaded spheres represent Br. -Which are trans-isomers?](https://storage.examlex.com/TB4939/11ea7a38_c9a8_78b7_aa4c_afb8c6bc3def_TB4939_00_TB4939_00_TB4939_00_TB4939_00.jpg) -Which are trans-isomers?
-Which are trans-isomers?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 141 - 160 of 185
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)