Exam 2: Atoms, Elements, and Compounds
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank.
There are _______mol is 72.36 g of citric acid, C6H8O7.
(Short Answer)
4.8/5  (35)
(35)
Convert the following number of moles into the corresponding mass. 5.22 mol Br
(Multiple Choice)
4.9/5  (33)
(33)
If the atom ratio of Na to N in a compound is 3:
1, the mole ratio of Na to N is 3:1.
(True/False)
5.0/5  (35)
(35)
How many moles of sulfur are there in a 0.685-g sample of sulfur?
(Multiple Choice)
4.9/5  (41)
(41)
If 1 mol of an element has a mass of 63.54 g, the symbol for this element is
(Multiple Choice)
4.9/5  (34)
(34)
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank.
There are _______mol is 54.05 g of boron.
(Short Answer)
4.8/5  (36)
(36)
Enter an integer number (1, 2, 3, ...) in the blank.
Sucrose (table sugar) has the formula C12H22O11. A sample of sucrose contains 2400 carbon atoms. How many formula units does this represent?
____________________formula units.
(Short Answer)
4.9/5  (45)
(45)
Enter the chemical symbol of the element in the blank.
Calcium forms the compound CaF2 . What other alkaline earth element with a smaller atomic mass would form a compound with a similar formula?
Chemical symbol:
_____________________.
(Short Answer)
4.8/5  (38)
(38)
Enter the chemical symbol of the element in the blank.
An element has the following electron arrangement.
shell 1:2 electrons shell 2:8 electrons shell 3:18 electrons shell 4:18 electrons shell 5: 4 electrons
The reactivity of this element would most closely resemble that of which element?
Chemical symbol:
_____________________
(Short Answer)
4.8/5  (44)
(44)
Enter the chemical symbol of the element in the blank.
An element in period 3 has the Lewis structure:
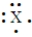 X should be replaced with what chemical symbol?
Chemical symbol:
_____________________.
X should be replaced with what chemical symbol?
Chemical symbol:
_____________________.
(Short Answer)
4.8/5  (33)
(33)
What is the mass number of an atom that is made up of 38 protons, 52 neutrons and 38 electrons?
(Multiple Choice)
4.8/5  (38)
(38)
What are the elements in the "A" columns of the period table called?
(Multiple Choice)
4.8/5  (24)
(24)
Consider the atomic view of a substance shown below.  This substance would be classified as a(n)
This substance would be classified as a(n)
(Multiple Choice)
4.8/5  (37)
(37)
Sodium chlorate, an ingredient in many common herbicides, has sodium, chlorine and oxygen atoms in the ratio 1: 1: 3, respectively. What is the formula unit for sodium chlorate?
(Multiple Choice)
4.8/5  (42)
(42)
How many moles of oxygen would be required to make 4 mol of the following compound? P4O10
(Multiple Choice)
4.9/5  (35)
(35)
An isotope of gallium consisting of 31 protons and 37 neutrons can be represented using the symbol shown below.
gallium-37
(True/False)
4.9/5  (34)
(34)
What is the meaning of the subscripts in the formula for ethyl alcohol, C2H5OH?
(Multiple Choice)
4.8/5  (37)
(37)
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank.
The mass of 7.00 mol of sodium chloride, NaCl, is _____________g.
(Short Answer)
4.7/5  (36)
(36)
Showing 41 - 60 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)