Exam 2: Atoms, Elements, and Compounds
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
An element has the following electron arrangement.
shell 1:2 electrons shell 2: 8 electrons shell 3:18 electrons shell 4:18 electrons shell 5:7 electrons
The Lewis structure for this element would be: 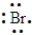
(True/False)
4.8/5  (34)
(34)
When a particular solid sample is examined under a microscope, it is observed that there are regions that are black and regions which are yellow. What type of matter is this sample?
(Multiple Choice)
4.8/5  (30)
(30)
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank.
The mass of 5.00 mol of oxygen atoms is _____________g.
(Short Answer)
4.9/5  (44)
(44)
An elements with an electron arrangement of:
shell 1:2 electrons shell 2:8 electrons shell 3:18 electrons shell 4: 18 electrons shell 5:7 electrons
would have 1 valence electron.
(True/False)
4.7/5  (41)
(41)
Sodium is a highly reactive metal and chlorine is a toxic gas, but when they come together the resulting material, sodium chloride (a white solid), is essential for life. Which of the following is true when sodium and chlorine are brought into contact with one another?
(Multiple Choice)
4.8/5  (35)
(35)
The image shown below represents an atomic view of a compound CsI.  What is the mass of one formula unit?
What is the mass of one formula unit?
(Multiple Choice)
4.9/5  (39)
(39)
Use the periodic table to determine about how many helium atoms (He) on the average would be needed to get close to the same mass as an oxygen atom (O).
(Multiple Choice)
4.8/5  (35)
(35)
Enter the chemical symbol in the blank for the element that has the electron arrangement given below.
shell 1: 2 electrons shell 2: 8 electrons shell 3: 5 electrons
Symbol for the element:
_____________________
(Short Answer)
5.0/5  (47)
(47)
Use one the following terms to complete the given statement.
salt
sugar
vegetable oil
When ____________________ is added to water a heterogeneous mixture forms.
(Short Answer)
4.9/5  (39)
(39)
Fill in each blank with the appropriate term from the list given below.
protons
neutrons
electrons
valence electrons
-14N and 15O have the same number of _____________________.
(Short Answer)
5.0/5  (38)
(38)
Enter an integer number (1, 2, 3, ...) in the blank.
Sucrose (table sugar) has the formula C12H22O11. How many oxygen atoms would be in 3 formula units of sucrose?
____________________oxygen atoms.
(Short Answer)
4.9/5  (36)
(36)
Which column of the periodic table is commonly called the halogens?
(Multiple Choice)
4.8/5  (35)
(35)
The atomic weight of phosphorus (P) is 30.91 amu or about 31 amu. This indicates that each P atom consists of 15 protons and 16 neutrons.
(True/False)
4.8/5  (43)
(43)
Elements in the same period of the periodic table generally show similar chemical behavior.
(True/False)
4.8/5  (34)
(34)
Showing 61 - 76 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)