Exam 13: Condensation and Hydrolysis Reactions
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
The formation of a copolymer requires that three different molecules react to form the polymer.
(True/False)
4.9/5  (34)
(34)
Consider the following compounds. 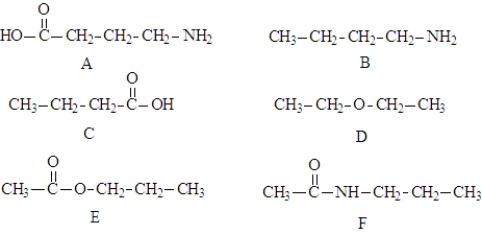 These compound may be involved in the following types of reaction.
amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list.More than one term may be correct.
-Structures E and F are the products of a(n) ____________________ reaction.
These compound may be involved in the following types of reaction.
amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list.More than one term may be correct.
-Structures E and F are the products of a(n) ____________________ reaction.
(Short Answer)
4.8/5  (32)
(32)
The following type of reaction is utilized by biological systems in obtaining large amounts of energy from food. 
(True/False)
4.9/5  (35)
(35)
The following represent several common nonsteriodal,anti-inflammatory drugs (NSAIDs)available over the counter. 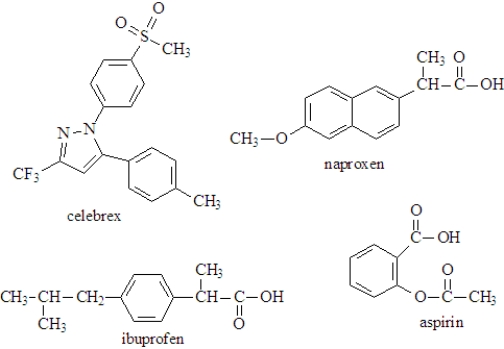 Fill the blanks with the appropriate term from the list below.More than one answer may be correct.
aspirin
ibuprofen
naproxen
celebrex
-_________________contains an ether functional group.
Fill the blanks with the appropriate term from the list below.More than one answer may be correct.
aspirin
ibuprofen
naproxen
celebrex
-_________________contains an ether functional group.
(Short Answer)
4.8/5  (35)
(35)
The following molecule was formed from the reaction of four alcohol molecules. 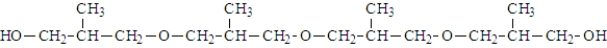
(True/False)
4.8/5  (33)
(33)
The following represent several common nonsteriodal,anti-inflammatory drugs (NSAIDs)available over the counter.  Fill the blanks with the appropriate term from the list below.More than one answer may be correct.
aspirin
ibuprofen
naproxen
celebrex
-_____________________would not increase the H3O+ concentration in the stomach.
Fill the blanks with the appropriate term from the list below.More than one answer may be correct.
aspirin
ibuprofen
naproxen
celebrex
-_____________________would not increase the H3O+ concentration in the stomach.
(Short Answer)
4.8/5  (44)
(44)
All of the bonds that hold the units together to form a polymer are:
(Multiple Choice)
5.0/5  (47)
(47)
Consider the polymer formed from equal numbers of the two amino acids glycine and alanine. Which of the following is not a property of this polymer?
(Multiple Choice)
4.8/5  (32)
(32)
Consider the following compounds.  These compound may be involved in the following types of reaction.
amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list.More than one term may be correct.
-Molecules of structure A could react with other molecules of A in a(n) ________________reaction to produce a very large molecule.
These compound may be involved in the following types of reaction.
amidation
esterification
saponification
condensation
hydrolysis
polymerization
Fill in the blanks with the appropriate term from the list.More than one term may be correct.
-Molecules of structure A could react with other molecules of A in a(n) ________________reaction to produce a very large molecule.
(Short Answer)
4.8/5  (33)
(33)
What are the products of the hydrolysis of the following compound when carried out at a physiological pH? 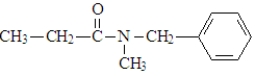
(Multiple Choice)
4.8/5  (41)
(41)
Draw the structure of the product of the condensation reaction of the following substances.
acetic acid and diethylamine
(Essay)
4.9/5  (41)
(41)
What mass in grams of ATP (C10H16N5O13P3) must be used to supply 4.26 kcal to the cells?
(Short Answer)
4.9/5  (51)
(51)
The following represent several common nonsteriodal,anti-inflammatory drugs (NSAIDs)available over the counter.  Fill the blanks with the appropriate term from the list below.More than one answer may be correct.
aspirin
ibuprofen
naproxen
celebrex
-______________________ can function as a proton acceptor.
Fill the blanks with the appropriate term from the list below.More than one answer may be correct.
aspirin
ibuprofen
naproxen
celebrex
-______________________ can function as a proton acceptor.
(Short Answer)
4.7/5  (44)
(44)
Showing 21 - 40 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)