Exam 2: Atoms, molecules, and Ions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Isotopes have the same number of ________ but different numbers of ________ in their nuclei.
(Short Answer)
4.8/5  (41)
(41)
Atoms of the same element always have the same number of ________ in their nuclei.
(Short Answer)
5.0/5  (44)
(44)
The bonding in NaI is ________,whereas the bonding in NH3 is ________.
(Short Answer)
4.8/5  (36)
(36)
The existence of neutrons in the nucleus of an atom was demonstrated by
(Multiple Choice)
4.9/5  (40)
(40)
What group of elements does the shaded area in the following periodic table indicate? 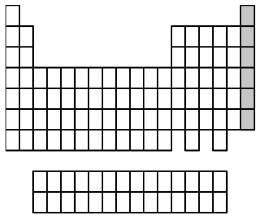
(Multiple Choice)
4.9/5  (40)
(40)
The number of atoms in 1 g of H is ________ (greater than,less than,the same as)the number of atoms in 12 g of C.
(Short Answer)
4.8/5  (43)
(43)
Assume that the mixture of substances in drawing (1)undergoes a chemical reaction.Which of the drawings (2)-(4)represents a product mixture that is consistent with the law of mass conservation? 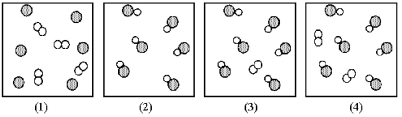
(Multiple Choice)
4.8/5  (41)
(41)
Which element indicated by letter in the following periodic table reacts rapidly with water to form an alkaline solution? 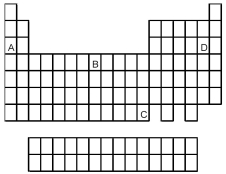
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following elements is a liquid at room temperature?
(Multiple Choice)
5.0/5  (39)
(39)
Which of the following represent isotopes? A: ![Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]](https://storage.examlex.com/TB4940/11ea7e2d_d096_728d_a2f7_0f2637b39515_TB4940_11.jpg) [ ] B:
[ ] B: ![Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]](https://storage.examlex.com/TB4940/11ea7e2d_d096_999e_a2f7_910be48eb3c7_TB4940_11.jpg) [ ] C:
[ ] C: ![Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]](https://storage.examlex.com/TB4940/11ea7e2d_d096_999f_a2f7_210203dd0fa0_TB4940_11.jpg) [ ] D:
[ ] D: ![Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]](https://storage.examlex.com/TB4940/11ea7e2d_d096_99a0_a2f7_c38f0b495a8d_TB4940_11.jpg) [ ]
[ ]
(Multiple Choice)
4.9/5  (45)
(45)
Showing 41 - 60 of 275
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)