Exam 2: Atoms, molecules, and Ions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Methane and oxygen react to form carbon dioxide and water.What mass of water is formed if 3.2 g of methane reacts with 12.8 g of oxygen to produce 8.8 g of carbon dioxide?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following elements has chemical properties similar to oxygen?
(Multiple Choice)
4.9/5  (28)
(28)
What is the standard isotope that is used to define the number of atoms in a mole?
(Multiple Choice)
4.8/5  (47)
(47)
What is the identity of element Q if the ion Q2+ contains 10 electrons?
(Multiple Choice)
4.7/5  (34)
(34)
The existence of electrons in atoms of all elements was demonstrated by
(Multiple Choice)
4.8/5  (35)
(35)
In which set do all elements tend to form anions in binary ionic compounds?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following elements has the least tendency to form an ion?
(Multiple Choice)
4.8/5  (33)
(33)
Gaseous elements characterized by low reactivity are found in group ________ of the periodic table.
(Multiple Choice)
4.9/5  (37)
(37)
If shaded and unshaded spheres represent atoms of different elements,as shown in drawing (1),which drawings (2)-(4)represent the law of multiple proportions? 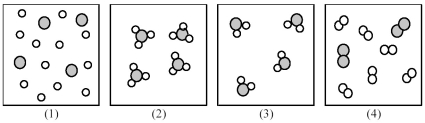
(Multiple Choice)
4.8/5  (36)
(36)
Give the molecular formula corresponding to the following ball-and-stick molecular representation of vitamin C (ascorbic acid)(gray = C,unshaded = H,black = O).In writing the formula,list the atoms in alphabetical order. 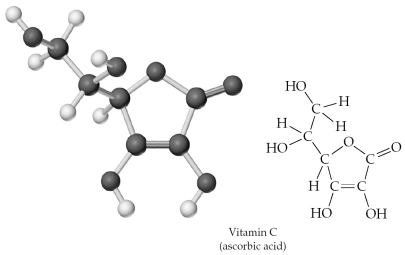
(Multiple Choice)
4.9/5  (38)
(38)
In a periodic table rows are called ________ and columns are called ________.
(Short Answer)
4.9/5  (38)
(38)
Butyric acid has the structural formula given below. 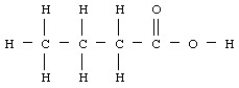 What is the molecular or chemical formula for butyric acid?
What is the molecular or chemical formula for butyric acid?
(Multiple Choice)
4.8/5  (42)
(42)
What group of elements does the shaded area in the following periodic table indicate? 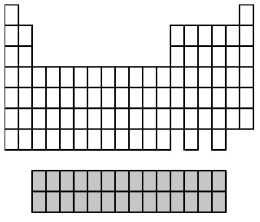
(Multiple Choice)
4.9/5  (40)
(40)
If shaded and unshaded spheres represent atoms of different elements,as shown in drawing (1),which drawings (2)-(4)represent the law of multiple proportions? 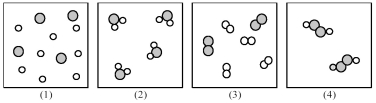
(Multiple Choice)
4.8/5  (37)
(37)
According to history,the concept that all matter is composed of atoms was first proposed by
(Multiple Choice)
4.8/5  (39)
(39)
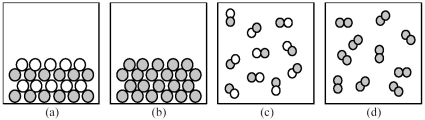 -If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents an ionic compound at room temperature and a pressure of 1 atm?
-If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents an ionic compound at room temperature and a pressure of 1 atm?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 141 - 160 of 275
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)