Exam 2: Atoms, molecules, and Ions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Use the periodic table below to answer the following questions. 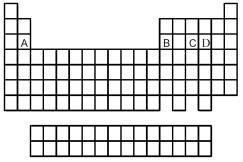 -Which is the correct formula of the binary fluoride of element B?
-Which is the correct formula of the binary fluoride of element B?
(Multiple Choice)
4.8/5  (40)
(40)
Which one of the following elements is a poor conductor of heat and electricity?
(Multiple Choice)
4.9/5  (30)
(30)
The subatomic particles contained in the nucleus of an atom are ________ and ________.
(Short Answer)
4.8/5  (34)
(34)
Sodium metal and water react to form hydrogen and sodium hydroxide.If 5.98 g of sodium react with water to form 0.26 g of hydrogen and 10.40 g of sodium hydroxide,what mass of water was consumed in the reaction?
(Multiple Choice)
4.9/5  (27)
(27)
Three atoms have the following properties. 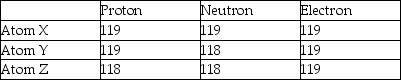 The elements X and Y are best described as
The elements X and Y are best described as
(Multiple Choice)
4.9/5  (35)
(35)
Which is most likely to form a binary oxide with the formula MO3 (where M = element A,B,C,or D)?
(Multiple Choice)
4.9/5  (35)
(35)
________ is a nonmetal that is a solid at room temperature.
(Multiple Choice)
4.8/5  (42)
(42)
Showing 261 - 275 of 275
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)