Exam 5: Periodicity and the Electronic Structure of Atoms
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
An orbital that as the appearance of a three-dimensional clover leaf has a quantum number l = ________.
(Short Answer)
4.8/5  (37)
(37)
How many valence electrons does a neutral polonium atom have?
(Multiple Choice)
4.8/5  (43)
(43)
A solution to the Schrödinger wave equation is a ________,or orbital,represented by the symbol Ψ,and the probability of finding an electron defined by Ψ within a given volume of space around the nucleus is ________.
(Short Answer)
4.8/5  (40)
(40)
An element in a ground state electron configuration has 4 electrons in the 4p orbitals.Which of the following statements can not describe the electron configurations in this atom?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following is not a valid set of quantum numbers?
(Multiple Choice)
4.8/5  (38)
(38)
Of the following,which atom has the smallest atomic radius?
(Multiple Choice)
4.9/5  (36)
(36)
Photochemists use electromagnetic radiation to initiate chemical reactions,often by providing the energy required to break bonds within a molecule.Lowering which of the following will result in electromagnetic radiation having more energy per photon?
(Multiple Choice)
4.9/5  (45)
(45)
What is the ground-state valence-shell electron configuration of the group of elements indicated by the shaded portion of the periodic table? 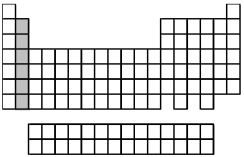
(Multiple Choice)
4.9/5  (39)
(39)
The smallest number that can be used for m or n in the Balmer-Rydberg equation is ________ and the largest number is ________.
(Short Answer)
4.8/5  (37)
(37)
Light can be made to have a higher intensity by raising its
(Multiple Choice)
4.7/5  (38)
(38)
The absorption of light of frequency 1.16 × 1011 Hz is required for CO molecules to go from the lowest rotational energy level to the next highest rotational energy level.Determine the energy for this transition in kJ/mol.(h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.7/5  (37)
(37)
Arrange the following spectral regions in order of increasing energy: infrared,microwave,ultraviolet,visible.
(Multiple Choice)
4.8/5  (37)
(37)
The spheres below represent atoms of Li,Be,B,and F (not necessarily in that order). 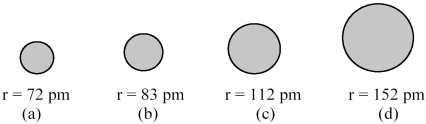 -Which one of these spheres represents an atom of F?
-Which one of these spheres represents an atom of F?
(Multiple Choice)
4.7/5  (44)
(44)
Two electromagnetic waves are represented below. 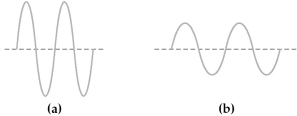 -Wave (a)has the
-Wave (a)has the
(Multiple Choice)
4.9/5  (42)
(42)
What is the ground-state electron configuration of tellurium?
(Multiple Choice)
4.9/5  (38)
(38)
The spheres below represent atoms of Sb,As,P,and N (not necessarily in that order). 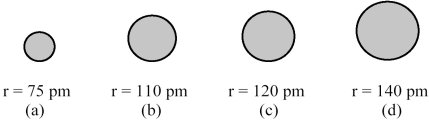 -Which one of these spheres represents an atom of As?
-Which one of these spheres represents an atom of As?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 101 - 120 of 168
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)