Exam 5: Periodicity and the Electronic Structure of Atoms
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
What is the de Broglie wavelength of a 300.g object moving at a velocity of 25.0 m/s (about 100 mph)?
(Multiple Choice)
4.9/5  (31)
(31)
The algebraic signs (+ and -)sometimes written within the lobes of orbitals are analogous to different ________ of a wave.
(Short Answer)
4.8/5  (33)
(33)
For a particular orbital,as one goes away from the nucleus along the z-axis,the probability density decreases to zero,then increases,and finally decreases without increasing a second time.This is consistent with a
(Multiple Choice)
4.9/5  (36)
(36)
Two electromagnetic waves are represented below. 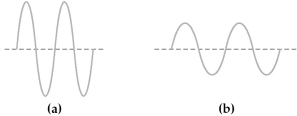 -Wave (b)has the
-Wave (b)has the
(Multiple Choice)
4.8/5  (33)
(33)
For a hydrogen atom,which electronic transition would result in the emission of a photon with the highest energy?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following elements would you predict to have an anomalous electron configuration?
(Multiple Choice)
4.8/5  (37)
(37)
Rank the following elements in order of increasing effective nuclear charge: Na,Mg,Cl,and S.
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following elements will have unpaired electrons in the ground state? Ca,Li,C,F
(Multiple Choice)
4.8/5  (36)
(36)
![-Which element,indicated by letter on the periodic table above,has the ground-state electron configuration [Ar]4s<sup>2</sup> 3d<sup>2</sup>?](https://storage.examlex.com/TB4940/11ea7e2d_d0b3_70a2_a2f7_ff1320f4b044_TB4940_00_TB4940_00.jpg) -Which element,indicated by letter on the periodic table above,has the ground-state electron configuration [Ar]4s2 3d2?
-Which element,indicated by letter on the periodic table above,has the ground-state electron configuration [Ar]4s2 3d2?
(Multiple Choice)
4.9/5  (38)
(38)
Which period of elements,indicated by letter on the periodic table,has electrons whose highest principal quantum number n is 5? 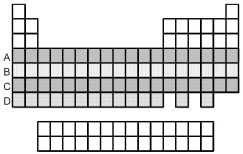
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following have their valence electrons in the same shell?
(Multiple Choice)
4.8/5  (41)
(41)
Molybdenum has an anomalous electron configuration.Write the electron configuration of Mo using shorthand notation.
(Multiple Choice)
4.8/5  (38)
(38)
Using shorthand notation,the electron configuration of Ni is ________.
(Short Answer)
4.9/5  (44)
(44)
According to the Balmer-Rydberg equation,transitions from n = 5 to m = 2 result in a photons of light that give rise to a spectral line with what color?
(Short Answer)
4.9/5  (39)
(39)
How many unpaired electrons are in an atom of Mt in its ground state?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 21 - 40 of 168
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)