Exam 5: Periodicity and the Electronic Structure of Atoms
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
The visible region of the electromagnetic radiation spectrum extends from ________ nm to ________ nm.
(Short Answer)
4.7/5  (43)
(43)
From the following list of observations,choose the one that most clearly supports the following conclusion by de Broglie: "Electrons have wave properties."
(Multiple Choice)
4.8/5  (35)
(35)
A person is most likely to experience serious tissue damage when exposed to which of the following forms of electromagnetic radiation?
(Multiple Choice)
4.9/5  (36)
(36)
Dentists employ light-cured materials to fill cavities.The wavelength of electromagnetic radiation used to photopolymerize restorative materials falls in the ultraviolet or visible region,depending on the instrument employed.Which of these wavelengths is in the UV region?
(Multiple Choice)
4.8/5  (39)
(39)
What is the first ionization energy for a hydrogen atom in the ground state? The Rydberg constant is 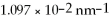 .
.
(Multiple Choice)
4.8/5  (45)
(45)
Within a given shell of a multielectron atom,the lower l for an orbital,the
(Multiple Choice)
4.8/5  (36)
(36)
Which orbital-filling diagram represents the anomalous ground state of chromium?
(Multiple Choice)
5.0/5  (30)
(30)
The amount of data that can be stored in an optical disc storage medium is related to the wavelength of the laser employed.Blu-Ray discs that are read by a laser having a wavelength of 405 nm have a much greater storage capacity than a DVD that is read by a 650 nm laser.The color of the 405 nm laser beam is ________,whereas the color of the 650 nm laser is ________.
(Short Answer)
4.9/5  (35)
(35)
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 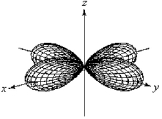
(Multiple Choice)
4.8/5  (32)
(32)
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 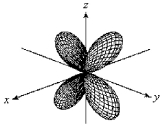
(Multiple Choice)
4.9/5  (37)
(37)
The ground-state electron configuration of the C4-,is the same as which noble gas?
(Short Answer)
4.8/5  (35)
(35)
The laser used to read Blu-Ray discs have a wavelength of 405 nm.405 nm photons have of an energy of ________ J,and a mole of 405 nm photons has an energy of ________ kJ/mol.
(Short Answer)
4.7/5  (41)
(41)
Which element has the ground-state electron configuration [Xe]6s2 4?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 41 - 60 of 168
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)