Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
A molecular model of NO3- is shown below.Based on the best Lewis electron-dot structure for NO3- and formal charge considerations,what is the predicted N-O bond order for each N-O bond? 
(Multiple Choice)
4.9/5  (31)
(31)
When an electron is added to the lowest unoccupied molecular orbital of N2,the electron is added to a(n)________ (antibonding,bonding)molecular orbital and the N-N bond order will ________ (decrease,increase).
(Short Answer)
4.8/5  (36)
(36)
Which drawing represents a π* antibonding molecular orbital for a homonuclear diatomic molecule?
(Multiple Choice)
4.8/5  (30)
(30)
The hybrid orbital used by carbon to overlap with hydrogen in C2H2 is ________.
(Short Answer)
4.8/5  (40)
(40)
Which molecular orbitals for homonuclear diatomic molecules are degenerate?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following should have the largest dipole moment?
(Multiple Choice)
4.8/5  (40)
(40)
The orbital hybridization on the central carbon atom in CH3CCH is
(Multiple Choice)
4.8/5  (37)
(37)
Which compound,shown with its dipole moment,is expected to exhibit the smallest percent ionic character?
(Multiple Choice)
4.8/5  (41)
(41)
Which best indicates the direction of the dipole moment in acetone, (CH3)2C=O? 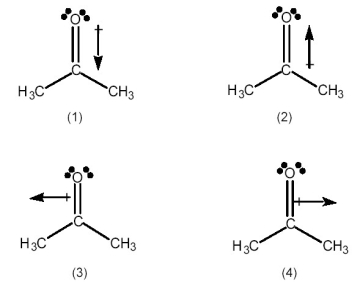
(Multiple Choice)
4.9/5  (40)
(40)
Which drawing best accounts for the polarity of methanol,CH3OH,and the bond polarities that make a major contribution to the overall molecular polarity? 
(Multiple Choice)
4.8/5  (25)
(25)
What are the bond angles in the following molecular model of NO3-? 
(Multiple Choice)
4.7/5  (32)
(32)
What is the geometry around the central atom in the following molecular model of SCl4? 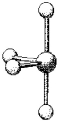
(Multiple Choice)
4.8/5  (41)
(41)
Which drawing represents a σ* antibonding molecular orbital for a homonuclear diatomic molecule?
(Multiple Choice)
4.9/5  (40)
(40)
What is the geometry around the central atom in the following molecular model of TeF5-? 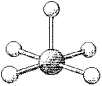
(Multiple Choice)
4.9/5  (42)
(42)
What is the geometry around the central atom in the following molecular model of XeF4? 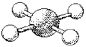
(Multiple Choice)
4.8/5  (25)
(25)
Electrostatic potential maps use color to portray the calculated electron distribution in a molecule.Atoms that are electron poor and carry a δ+ charge are shown in blue.Atoms that are electron rich and carry a δ- charge are shown in red.Atoms with little or no charge are shown in green.The electrostatic potential map of CH3Cl below should show 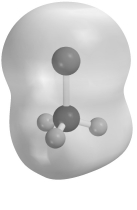
(Multiple Choice)
4.8/5  (36)
(36)
What are the bond angles in the following molecular model of SI6? 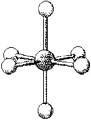
(Multiple Choice)
4.8/5  (36)
(36)
Showing 161 - 180 of 205
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)