Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Shown below is a model of SF6 having an orientation in which one or more atoms are hidden from view.What is the indicated bond angle? 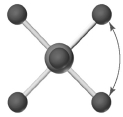
(Multiple Choice)
4.9/5  (29)
(29)
Which atomic orbitals are involved in bonding and which as lone pair orbitals for N2H2? H - 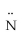 =
= 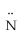 - H
- H
(Multiple Choice)
4.9/5  (36)
(36)
What are the bond angles in the following molecular model of SiCl4? 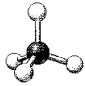
(Multiple Choice)
4.8/5  (39)
(39)
The HI bond has a length of 161 pm and 4.92% ionic character.What is the experimental dipole moment of HI?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following compounds has the highest boiling point?
(Multiple Choice)
4.8/5  (39)
(39)
Shown below is a model of PCl5 having an orientation in which one or more atoms are hidden from view.What is the indicated bond angle? 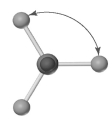
(Multiple Choice)
5.0/5  (38)
(38)
What is the geometry around the central atom in the following molecular model of SO2? 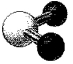
(Multiple Choice)
4.8/5  (39)
(39)
LiH has an experimental dipole moment,μ = 6.00 D.If LiH were 100% ionic,the distance between positive and negative charges would be 161 pm.What is the percent ionic character in the LiH bond?
(Short Answer)
4.9/5  (33)
(33)
Which drawing represents the lowest energy unoccupied molecular orbital in the N2 molecule in its ground state?
(Multiple Choice)
4.8/5  (47)
(47)
What are the bond angles in the following molecular model of PH3? 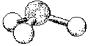
(Multiple Choice)
5.0/5  (37)
(37)
What are the bond angles in the following molecular model of PCl5? 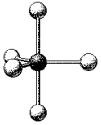
(Multiple Choice)
4.9/5  (41)
(41)
What are the bond angles in the following molecular model of PF6-? 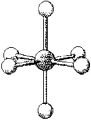
(Multiple Choice)
4.8/5  (32)
(32)
What are the bond angles in the following molecular model of H3O+? 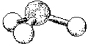
(Multiple Choice)
4.8/5  (52)
(52)
Showing 81 - 100 of 205
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)