Exam 4: Subatomic Particles
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Visible light has a frequency range between 7 × 1014 Hz and 4 × 1014 Hz. Which frequency of light would have the most energy?
(Multiple Choice)
4.8/5  (39)
(39)
Which contributes more to an atom's mass: electrons or protons? Which contributes more to an atom's size?
(Multiple Choice)
5.0/5  (35)
(35)
Which of the following is not a form of electromagnetic radiation?
(Multiple Choice)
4.9/5  (33)
(33)
The following statement describes which subatomic particle best? It does not have an electrical charge.
(Multiple Choice)
4.9/5  (36)
(36)
If an element has 10 protons and 11 neutrons and 10 electrons, which expression correctly identifies the element?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following could best be represented by a physical model?
(Multiple Choice)
4.9/5  (35)
(35)
If a neutral element has the following chemical symbol, how many electrons does it have? 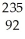 U
U
(Multiple Choice)
4.9/5  (31)
(31)
A beam of protons and a beam of neutrons of the same energy are both harmful to living tissue. The beam of neutrons, however, is less harmful. Why?
(Multiple Choice)
4.8/5  (34)
(34)
In the following diagram, which of the following is the measurement of one wavelength? 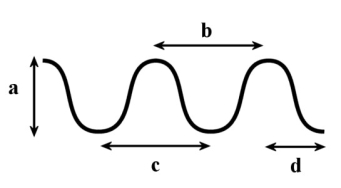
(Multiple Choice)
4.8/5  (39)
(39)
About how many times larger is a bacterium compared to an atom?
(Multiple Choice)
4.8/5  (41)
(41)
If you remove two protons and two neutrons from a gold atom (Au), what new element is formed?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following diagrams best represents the size of the atomic nucleus relative to the size of the atom? 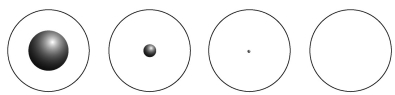 A B C D
A B C D
(Multiple Choice)
4.8/5  (36)
(36)
If an element has 18 protons and 20 neutrons and 18 electrons, which expression correctly identifies the element?
(Multiple Choice)
4.9/5  (34)
(34)
What is the difference between radiation and electromagnetic radiation?
(Multiple Choice)
4.9/5  (51)
(51)
What was Niels Bohr's explanation for the observation of atomic spectra?
(Multiple Choice)
4.8/5  (38)
(38)
Oxygen, O, (number 8), sulfur, S, (number 16), and selenium, Se, (number 34) have such similar chemical properties because ________.
(Multiple Choice)
5.0/5  (42)
(42)
Showing 41 - 60 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)