Exam 4: Subatomic Particles
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Which of the following concepts underlies all the others: ionization energy, effective nuclear charge, atomic size.
(Multiple Choice)
4.8/5  (46)
(46)
If an element has 9 protons and 10 neutrons and 9 electrons, which expression correctly identifies the element?
(Multiple Choice)
4.8/5  (42)
(42)
What prompted early scientists to propose that the ray of the cathode ray tube was due to the cathode?
(Multiple Choice)
4.7/5  (35)
(35)
What color do you see when you close your eyes while in a dark room? Explain.
(Multiple Choice)
4.8/5  (36)
(36)
Neon, Ne, (number 10) has a relatively large effective nuclear charge, yet it cannot attract any additional electrons because ________.
(Multiple Choice)
4.9/5  (34)
(34)
How might you distinguish a sodium-vapor street lamp from a mercury-vapor street lamp?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following experiments helped to determine the charge of an electron and (indirectly) its mass?
(Multiple Choice)
4.7/5  (39)
(39)
The following statement describes which subatomic particle best? It is located outside of the nucleus.
(Multiple Choice)
4.7/5  (40)
(40)
In Rutherford's gold foil experiment, alpha particles ________.
(Multiple Choice)
4.9/5  (36)
(36)
If the particles of a cathode ray had a greater electric charge, the ray passing through a magnetic field would bend ________.
(Multiple Choice)
4.9/5  (35)
(35)
Which experiences a greater effective nuclear charge, an electron in the outer shell of neon (atomic number 10) or an electron in the outer shell of sodium (atomic number 11)?
(Multiple Choice)
4.9/5  (35)
(35)
What do the components of a conceptual model have in common?
(Multiple Choice)
4.8/5  (42)
(42)
The isotope lithium-7 has a mass of 7.0160 atomic mass units, and the isotope lithium-6 has a mass of 6.0151 atomic mass units. Given the information that 92.58 percent of all lithium atoms found in nature are lithium-7 and 7.42 percent are lithium-6, calculate the atomic mass of lithium, Li (atomic number 3).
(Multiple Choice)
4.8/5  (28)
(28)
Rutherford assumed that the atomic nucleus was positively charged because ________.
(Multiple Choice)
5.0/5  (39)
(39)
Using the following generic atom description, choose the correct method for determining the number of neutrons. 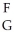 X
X
(Multiple Choice)
4.8/5  (43)
(43)
What do the electron configurations for all the group 18 noble gases have in common?
(Multiple Choice)
4.8/5  (38)
(38)
Evidence for the existence of neutrons did not come until many years after the discoveries of the electron and the proton. Give a possible explanation.
(Multiple Choice)
4.9/5  (33)
(33)
Showing 81 - 100 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)