Exam 4: Subatomic Particles
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Which of the following best describes a conceptual model of an atom?
(Multiple Choice)
4.7/5  (28)
(28)
An electron in an outermost shell has more energy than one in an innermost shell because ________.
(Multiple Choice)
4.9/5  (24)
(24)
If a neutral element has the following chemical symbol, how many electrons does it have? 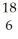 O
O
(Multiple Choice)
4.7/5  (44)
(44)
Since atoms are mostly empty space, why don't objects pass through one another?
(Multiple Choice)
4.8/5  (32)
(32)
List the following atoms in order of increasing atomic size: Thallium, Tl; Germanium, Ge; Tin, Sn; Phosphorus, P.
(Multiple Choice)
4.8/5  (38)
(38)
An electron in the outermost shell of which group 1 element experiences the greatest effective nuclear charge?
(Multiple Choice)
4.8/5  (34)
(34)
If a neutral element has 8 neutrons and 7 electrons, which expression correctly identifies the element?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following experiments helped to determine that most of an atom is actually empty space except for a very small positive core?
(Multiple Choice)
4.7/5  (37)
(37)
What is the effective nuclear charge for an electron in the outermost shell of a fluorine atom, F (atomic number 9)? How about one in the outermost shell of a sulfur atom, S (atomic number 16)?
(Multiple Choice)
4.8/5  (37)
(37)
Would you use a physical model or a conceptual model to describe the following: a gold coin, dollar bill, car engine, air pollution, virus, spread of sexually transmitted disease?
(Multiple Choice)
4.8/5  (42)
(42)
An element found in another galaxy exists as two isotopes. If 80.0 percent of the atoms have an atomic mass of 80.00 amu and the other 20.0 percent have an atomic mass of 82.00 amu, what is the atomic mass of the element?
(Multiple Choice)
4.8/5  (41)
(41)
Some older cars vibrate loudly when driving at particular speeds. For example, at 65 mph the car may be most quiet, but at 60 mph the car rattles uncomfortably. How is this analogous to the quantized energy levels of an electron in an atom?
(Multiple Choice)
4.8/5  (33)
(33)
An element has two different isotopes: one that weighs 65 amu and another that weighs 67 amu. If the average atomic mass of all the isotopes is 66.5 amu, what can be said about the relative abundance of the isotopes?
(Multiple Choice)
4.7/5  (37)
(37)
How many electrons are there in the third shell of sodium, Na (atomic number 11)?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 101 - 120 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)