Exam 10: Acids and Bases in Our Environment
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
What happens to the pH of an acidic solution as water is added?
(Multiple Choice)
4.9/5  (36)
(36)
In the following buffer system, what happens to the concentration of the highlighted molecule if you add acid in the form of H3O+? HF + NaF + H2O ⇌ F- + H3O+ + Na+
(Multiple Choice)
4.9/5  (39)
(39)
Which of the above illustrations shows a basic aqueous solution?
(Multiple Choice)
4.9/5  (33)
(33)
Would it be easier or harder for a water molecule to donate a hydrogen ion if its polar H-O bond were even more polar? Why?
(Multiple Choice)
4.7/5  (37)
(37)
What do the brackets in the following equation represent? [H3O+] × [OH-] = Kw
(Multiple Choice)
4.9/5  (24)
(24)
According to the following reaction, which molecule is acting as an acid? H2O + NH3 → OH- + NH4+
(Multiple Choice)
4.8/5  (34)
(34)
If the rate at which we produce CO2 were to stop rising, the levels of CO2 in the atmosphere would ________.
(Multiple Choice)
4.8/5  (38)
(38)
According to the following reaction, which molecule is acting as an acid? OH- + NH4+ → H2O + NH3
(Multiple Choice)
4.8/5  (41)
(41)
Hydrogen chloride is added to a buffer solution of ammonia, N 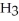 , and ammonium chloride, N
, and ammonium chloride, N 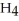 ⁺Cl⁻. What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
⁺Cl⁻. What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
(Multiple Choice)
4.7/5  (44)
(44)
Sodium hydroxide, NaOH, is a strong base, which means that it readily accepts hydrogen ions. What products are formed when sodium hydroxide accepts a hydrogen ion from a water molecule?
(Multiple Choice)
4.8/5  (37)
(37)
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-5M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.8/5  (42)
(42)
Which of the above illustrations shows an acidic aqueous solution?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following statements about strong and weak bases is not true?
(Multiple Choice)
5.0/5  (32)
(32)
According to the following reaction, which molecule is acting as a base? OH- + NH4+ → H2O + NH3
(Multiple Choice)
4.8/5  (38)
(38)
Showing 121 - 135 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)