Exam 10: Acids and Bases in Our Environment
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
The overall rate at which humans are producing carbon dioxide is ________.
(Multiple Choice)
4.8/5  (41)
(41)
If the pH of a solution was 7 and you were to increase the hydronium ion concentration 1000x, what would the pH be?
(Multiple Choice)
4.8/5  (37)
(37)
For the following acid-base reaction, identify what compound is formed in the space marked. HNO3 + KOH ⇌ ???? + H2O
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following statements about strong and weak acids is not true?
(Multiple Choice)
4.9/5  (37)
(37)
Does the hydride ion, H⁻, tend to behave as an acid or a base?
(Multiple Choice)
5.0/5  (47)
(47)
If the pH of a solution is 10, what is the hydroxide ion concentration?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following reactions illustrates an amphoteric compound?
(Multiple Choice)
4.9/5  (46)
(46)
An acid and a base react to form a salt, which consists of positive and negative ions. Which forms the positive ions: the acid or the base? Which forms the negative ions?
(Multiple Choice)
4.7/5  (38)
(38)
If you had a 1 M solution of a weak acid what would be its pH?
(Multiple Choice)
4.7/5  (35)
(35)
The main component of bleach is sodium hypochlorite, NaOCl, which consists of sodium ions, Na⁺, and hypochlorite ions, 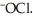 What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
(Multiple Choice)
4.9/5  (40)
(40)
Which should be a stronger base: ammonia, 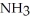 , or trifluoronitrogen,
, or trifluoronitrogen, 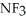 ? Why?
? Why? 
(Multiple Choice)
4.8/5  (44)
(44)
What happens to the pH of a 1M solution of hydrochloric acid, HCl, as carbon dioxide gas is bubbled into it?
(Multiple Choice)
4.8/5  (33)
(33)
As the pH increases, the hydroxide ion concentration ________.
(Multiple Choice)
4.9/5  (42)
(42)
How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Why? 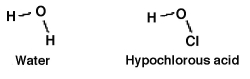
(Multiple Choice)
4.9/5  (34)
(34)
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following items would you use to make an acidic buffer solution?
(Multiple Choice)
5.0/5  (42)
(42)
Why might an area with a large amount of limestone (CaCO3) be less susceptible to acid rain?
(Multiple Choice)
4.9/5  (32)
(32)
When a hydronium ion concentration equals 1 × 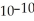 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.9/5  (43)
(43)
Qualitatively, what happens to the hydroxide ion concentration if you decrease the hydronium ion concentration?
(Multiple Choice)
4.9/5  (32)
(32)
Showing 101 - 120 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)