Exam 10: Acids and Bases in Our Environment
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
When the pH of a solution is 1, the concentration of hydronium ions is 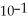 M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
(Multiple Choice)
4.8/5  (39)
(39)
At what point will a buffer solution cease to moderate changes in pH?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following statements describes an acidic solution?
(Multiple Choice)
4.9/5  (41)
(41)
Some molecules are able to stabilize a negative charge by passing it from one atom to the next by a flip-flopping of double bonds. This occurs when the negative charge is one atom away from an oxygen double bond as follows. Note that the curved arrows indicate the movement of electrons: 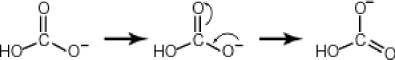 Why then is sulfuric acid so much stronger an acid than carbonic acid?
Why then is sulfuric acid so much stronger an acid than carbonic acid? 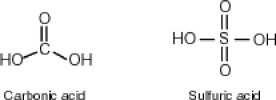
(Multiple Choice)
4.8/5  (44)
(44)
What results when water, 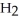 O, reacts with sulfur dioxide, S
O, reacts with sulfur dioxide, S 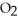 ?
?
(Multiple Choice)
4.9/5  (34)
(34)
According to the following reaction, which molecule is acting as an acid? H3O+ + HSO4- → H2O + H2SO4
(Multiple Choice)
4.8/5  (31)
(31)
When the hydronium ion concentration equals 1 mole per liter, what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.8/5  (38)
(38)
Cutting back on the pollutants that cause acid rain is one solution to the problem of acidified lakes. Suggest another.
(Multiple Choice)
4.9/5  (34)
(34)
Sometimes an individual going through a traumatic experience cannot stop hyperventilating. In such a circumstance, it is recommended that the individual breath into a paper bag or cupped hands as a useful way to avoid an increase in blood pH, which can cause the person to pass out. Explain how this works.
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following statements is not true about a neutralization reaction?
(Multiple Choice)
4.7/5  (30)
(30)
What is the hydroxide ion concentration in an aqueous solution where the pH = 5?
(Multiple Choice)
4.8/5  (40)
(40)
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
(Multiple Choice)
4.9/5  (38)
(38)
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-11M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.8/5  (39)
(39)
As the hydronium ion concentration increases, the pH ________.
(Multiple Choice)
4.8/5  (39)
(39)
Why do we use the pH scale to indicate the acidity of a solution rather than simply stating the concentration of hydronium ions?
(Multiple Choice)
4.8/5  (28)
(28)
For the following acid-base reaction, identify what is formed in the space marked. HF + KOH ⇌ ???? + H2O
(Multiple Choice)
5.0/5  (37)
(37)
Showing 61 - 80 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)