Exam 10: Acids and Bases in Our Environment
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Pour vinegar onto beach sand from the Caribbean and the result is a lot of froth and bubbles. Pour vinegar onto beach sand from California, however, and nothing happens. Why?
(Multiple Choice)
4.9/5  (31)
(31)
Adding more carbon dioxide to the atmosphere causes the concentration of carbonates in the ocean to ________.
(Multiple Choice)
4.9/5  (32)
(32)
What is the relationship between the hydroxide ion and a water molecule?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following compounds could never act as an acid?
(Multiple Choice)
4.7/5  (34)
(34)
Identify the acid or base behavior of each substance in these reactions: HS 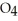
 +
+ 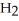 O ⇌ O
O ⇌ O 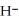 +
+ 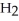 S
S 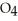
(Multiple Choice)
4.8/5  (29)
(29)
If you had a 1 M solution of a strong acid what would be its pH?
(Multiple Choice)
4.7/5  (41)
(41)
Acetic acid, shown below, has 4 hydrogen atoms-one bonded to an oxygen and three bonded to a carbon. When this molecule behaves as an acid, it donates only the hydrogen bonded to the oxygen. The hydrogens bonded to the carbon remain intact. Why? 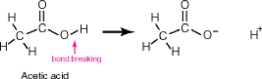
(Multiple Choice)
4.9/5  (35)
(35)
For the following acid-base reaction, identify what is formed in the space marked. H2SO4 + KOH ⇌ KHSO4 + ???
(Multiple Choice)
4.8/5  (35)
(35)
If you were to increase the pH of rain water the concentration of dissolved CO2 would ________.
(Multiple Choice)
4.8/5  (41)
(41)
If you had a 1 M solution of a strong base what would be its pH?
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following statements describes a basic solution?
(Multiple Choice)
4.9/5  (42)
(42)
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
(Multiple Choice)
4.8/5  (40)
(40)
Sodium bicarbonate, NaHC 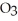 , is the active ingredient of baking soda. Compare its structure carefully with the weak acids and bases presented in this chapter and explain how this compound by itself in solution (with no other components) moderates changes in pH.
, is the active ingredient of baking soda. Compare its structure carefully with the weak acids and bases presented in this chapter and explain how this compound by itself in solution (with no other components) moderates changes in pH. 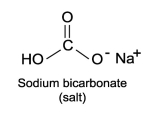
(Multiple Choice)
4.8/5  (38)
(38)
Sodium hydroxide, NaOH, is a very strong base. If a concentrated solution of this base were to spill on a latex glove you were wearing, it would feel like regular water. If the solution were to land directly on your skin, however, it would feel very slippery. Why?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following statements about strong or weak bases is true?
(Multiple Choice)
4.8/5  (27)
(27)
In the following buffer system, what happens to the concentration of the highlighted molecule if you add base in the form of OH-? HF + NaF + H2O ⇌ F- + H3O+ + Na+
(Multiple Choice)
4.8/5  (41)
(41)
According to the following reaction, which molecule is acting as an acid? H2O + H2SO4 → H3O+ + HSO4-
(Multiple Choice)
4.8/5  (39)
(39)
Showing 21 - 40 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)