Exam 1: Basic Concepts of Chemistry
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
A mass of 10 g of table salt dissolves in water to form a(n)________ mixture (i.e.,a mixture that is uniform throughout).
(Short Answer)
4.8/5  (47)
(47)
Which of the following statements concerning the kinetic-molecular theory of matter is/are CORRECT?
1)Particles in a liquid vibrate back and forth about an average position.
2)Particles in a solid are packed closely together,but are not confined to specific positions.
3)Particles in a gas fly about randomly,colliding with themselves and the walls of their container.
(Multiple Choice)
4.8/5  (32)
(32)
The law of ________ states that the total energy of the universe is constant.
(Short Answer)
4.8/5  (39)
(39)
Use the figures below
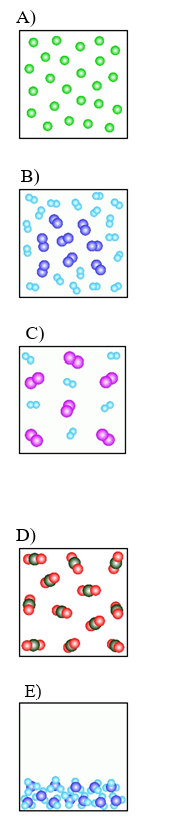
A)  B)
B)  C)
C) 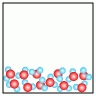 D)
D)  E)
E) 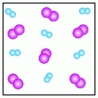 -Which of the above figures represents a ?
-Which of the above figures represents a ?
(Multiple Choice)
4.8/5  (31)
(31)
Use the pictures below
 -Which of the above figures represents a ?
-Which of the above figures represents a ?
(Multiple Choice)
4.8/5  (40)
(40)
A number of the heaviest elements on the periodic table are named for famous scientists.Element number 106 was most likely named for which famous scientist?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following are extensive properties: mass,volume,and/or density?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following statements is/are CORRECT?
1)Atoms are the smallest particles of an element that retain the element's chemical properties.
2)Substances composed of only one type of atom are classified as elements.
3)Of the 118 known elements,only 48 occur naturally.
(Multiple Choice)
4.8/5  (32)
(32)
Potential energy possessed by water at the top of a waterfall is known as ________ energy.
(Short Answer)
4.9/5  (36)
(36)
Which of the following are likely to form a homogeneous mixture?
1) milk and ice cream blended together with chocolate syrup
2) an egg combined with milk and mixed with a whisk
3)1 gram table salt combined with 250 mL of water
(Multiple Choice)
4.8/5  (39)
(39)
Which one of the following statements is not a comparison of physical properties?
(Multiple Choice)
4.7/5  (36)
(36)
An electrically charged atom or group of atoms is a(n)________.
(Multiple Choice)
4.9/5  (32)
(32)
To ensure integrity in science,experimental results should be ________ and reported in sufficient detail that the experiment can be repeated by others.
(Short Answer)
4.8/5  (31)
(31)
Showing 21 - 40 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)