Exam 7: The Structure of Atoms
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
For which of the following transitions would a hydrogen atom absorb a photon with the longest wavelength?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
E
For a proton (mass = 1.673 10-27 kg)moving with a velocity of 2.83 104 m/s,what is the de Broglie wavelength (in pm)?
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
C
What is the binding energy of an electron in a photosensitive metal (in kJ/mol)if the longest wavelength of light that can eject electrons from the metal is 205 nm?
Free
(Multiple Choice)
4.7/5  (44)
(44)
Correct Answer:
B
The ________ quantum number is given the symbol 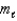 .This quantum number is related to the orientation in space of an orbital.
.This quantum number is related to the orientation in space of an orbital.
(Short Answer)
4.9/5  (29)
(29)
Which of the following orbital boundary surfaces is a representation of a 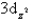 orbital?
orbital?
(Multiple Choice)
4.8/5  (52)
(52)
What is the energy of a photon of electromagnetic radiation with a frequency of 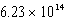 Hz?
Hz?
(Multiple Choice)
4.7/5  (47)
(47)
Which of the following is a representation of a 3dxz orbital?
(Multiple Choice)
4.8/5  (34)
(34)
A ________,designated by the Greek symbol ,describes the wave behavior of an electron in an atom.
(Short Answer)
4.9/5  (29)
(29)
What is the energy per mole of photons of light with a frequency of 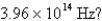
(Multiple Choice)
4.8/5  (41)
(41)
What is the wavelength of a photon having a frequency of 11.7 THz? (1 THz = 1015 Hz)
(Multiple Choice)
4.8/5  (32)
(32)
The contribution for which de Broglie is best remembered in modern science is
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following statements is/are CORRECT?
1)A diamagnetic substance is strongly attracted to a magnetic field.
2)Substances that retain their magnetism after they are withdrawn from a magnetic field are called ferromagnetic.
3)Most transition metals and all lanthanide metals are ferromagnetic.
(Multiple Choice)
4.7/5  (35)
(35)
The n = ____ shell is the lowest that may contain s-orbitals.
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following sets of quantum numbers (n,l,ml, ms)is not permissible?
(Multiple Choice)
4.8/5  (37)
(37)
What evidence does the photoelectric effect provide that photons are not only waves?
(Essay)
4.9/5  (34)
(34)
What is the frequency of gamma ray radiation that has a wavelength of 11.4 pm?
(Multiple Choice)
4.7/5  (44)
(44)
What is the total number of orbitals found in the n = 4 shell?
(Multiple Choice)
4.8/5  (33)
(33)
A possible value of the magnetic quantum number 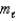 for a 5d electron is
for a 5d electron is
(Multiple Choice)
4.8/5  (40)
(40)
Showing 1 - 20 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)