Exam 20: Principles of Reactivity: Electron Transfer Reactions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
In the following electrochemical cell,what is the reduction half reaction?
Cu(s)| Cu2+(aq)|| Fe3+(aq),Fe2+(aq)| Pt(s)
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
A
The use of electrical energy to produce chemical change is known as ________.An example of this process is the reduction of NaCl( 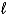 )to produce Na(s).
)to produce Na(s).
Free
(Short Answer)
4.8/5  (36)
(36)
Correct Answer:
electrolysis
What is the correct cell notation for a voltaic cell based on the reaction below?
Cu2+(aq)+ Fe(s) Cu(s)+ Fe2+(aq)
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
E
The unit for electromotive force,emf,is the Volt.A Volt is equal to
(Multiple Choice)
5.0/5  (42)
(42)
What is the correct cell notation for a cell in which the hydrogen electrode is the anode and the cathode half-reaction is
Pb4+(aq)+ 2e- Pb2+(aq).
(Multiple Choice)
4.8/5  (37)
(37)
Write a balanced chemical equation for the overall reaction represented by the cell notation below.Mn(s)| Mn2+(aq)|| Zn2+(aq)| Zn(s)
(Multiple Choice)
4.9/5  (32)
(32)
The following reaction occurs spontaneously.
2 Fe(s)+ 3 Cl2(aq) 2 Fe3+(aq)+ 6 Cl-(aq)
Write the balanced oxidation half-reaction.
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following statements is/are CORRECT?
1)A nickel-cadmium battery is an example of a secondary or rechargeable battery,often used in rechargeable cordless appliances.
2)Hydrogen-oxygen fuel cells use the heat of combustion of hydrogen to recharge lead storage batteries.
3)LeClanché cells are the most efficient rechargeable batteries,but they are rarely used due their high cost of production.
(Multiple Choice)
4.9/5  (37)
(37)
Calculate Ecell for the following electrochemical cell at 25°C given that the standard cell potential,
E°cell,is 0.460 V.
Cu(s)| Cu2+(aq,0.012 M)|| Ag+(aq,0.17 M)| Ag(s)
(Multiple Choice)
4.9/5  (37)
(37)
Al3+ is reduced to Al(s)at an electrode.If a current of 2.75 ampere is passed for 36 hours,what mass of aluminum is deposited at the electrode? Assume 100% current efficiency.
(Multiple Choice)
4.8/5  (45)
(45)
An SHE electrode has been assigned a standard reduction potential,E ,of 0.00 Volts.Which reaction occurs at this electrode?
(Multiple Choice)
4.9/5  (44)
(44)
Write balanced reduction and oxidation half-reactions for the processes that occur at the cathode and anode of a fuel cell used aboard NASA's space shuttles.
(Essay)
4.7/5  (33)
(33)
Calculate 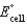 for the electrochemical cell below,
Ag(s)| AgCl(s)| Cl-(aq,1.0 M)|| Cu2+(aq,1.0 M)| Cu(s)
Given the following standard reduction potentials.
Cu2+(aq)+ 2 e- Cu(s) E = +0.337 V
AgCl(s)+ e- Ag(s)+ Cl-(aq) E = +0.222 V
for the electrochemical cell below,
Ag(s)| AgCl(s)| Cl-(aq,1.0 M)|| Cu2+(aq,1.0 M)| Cu(s)
Given the following standard reduction potentials.
Cu2+(aq)+ 2 e- Cu(s) E = +0.337 V
AgCl(s)+ e- Ag(s)+ Cl-(aq) E = +0.222 V
(Multiple Choice)
4.9/5  (35)
(35)
What is the standard cell potential ( 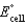 )for the following below,
2Ag(s)+ Pb2+(aq) 2Ag+(aq)+ Pb(s)
Given the following standard reduction potentials.
Ag+(aq)+ e- Ag(s) E =0.8 V
Pb2+(aq)+2e- Pb(s) E =-0.126 V
)for the following below,
2Ag(s)+ Pb2+(aq) 2Ag+(aq)+ Pb(s)
Given the following standard reduction potentials.
Ag+(aq)+ e- Ag(s) E =0.8 V
Pb2+(aq)+2e- Pb(s) E =-0.126 V
(Multiple Choice)
4.7/5  (35)
(35)
Consider the following half-reactions:
Cl2(g)+ 2 e- 2 Cl-(aq) E = +1.36 V
Ag+(aq)+ e- Ag(s) E = +0.80 V
Cu2+(aq)+ 2 e- Cu(s) E = +0.34 V
Sn2+(aq)+ 2 e- Sn(s) E = -0.14 V
Al3+(aq)+ 3 e- Al(s) E = -1.66 V
Which of the above elements or ions will reduce Cu2+(aq)?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following are standard conditions for an electrochemical cell?
1)Solutes in aqueous solution has a concentration of 1 M.
2)Gaseous reactants or products have a pressure of 1 bar.
3)Solids are present in quantities of 1 mole.
(Multiple Choice)
5.0/5  (33)
(33)
For the cell reaction 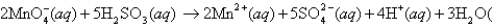 l )
The standard cell potential is 1.34 V.To determine the cell potential at nonstandard conditions,what is the value that should be used for n in the Nernst equation?
l )
The standard cell potential is 1.34 V.To determine the cell potential at nonstandard conditions,what is the value that should be used for n in the Nernst equation?
(Multiple Choice)
4.8/5  (34)
(34)
Calculate E°cell for the cell for the reaction below,
2Cr(s)+ 3Sn4+(aq) 3Sn2+(aq)+2Cr3+(aq)
Given the following standard reduction potentials.
Cr3+(aq)+ 3e- Cr(s) E = -0.74 V
Sn4+(aq)+ 2e- Sn2+(aq) E = 0.15V
(Multiple Choice)
4.8/5  (41)
(41)
Given the following two half-reactions,write the overall reaction in the direction in which it is spontaneous and calculate the standard cell potential.
Pb2+(aq)+ 2 e- Pb(s) E = -0.126 V
Fe3+(aq)+ e- Fe2+(s) E = +0.771 V
(Multiple Choice)
4.8/5  (40)
(40)
Showing 1 - 20 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)