Exam 3: Atoms, molecules, and Ions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
The element chlorine has two stable isotopes,chlorine-35 with an atomic mass of 34.97 u and chlorine-37 with an atomic mass of 36.97 u.From the atomic weight found on the periodic table,one can conclude that:
(Multiple Choice)
4.8/5  (43)
(43)
What is the mass percent of each element in sulfuric acid,H2SO4?
(Multiple Choice)
4.9/5  (33)
(33)
What is the correct formula for potassium dihydrogen phosphate?
(Multiple Choice)
4.9/5  (34)
(34)
In which ionic compound,NaBr or KBr,is the force of attraction between anions and cations stronger?
(Essay)
4.7/5  (32)
(32)
Which of the following statements concerning atomic structure is/are correct?
1)Neutrons and electrons are found in space as a cloud around the nucleus.
2)The nucleus contains all the positive charge of an atom.
3)Electrons surround the nucleus and account for the majority of an atom's volume.
(Multiple Choice)
4.8/5  (42)
(42)
What is the mass percent of chlorine in magnesium chloride?
(Multiple Choice)
4.9/5  (47)
(47)
Which of the following atomic symbols represents an isotope of 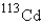 ?
?
(Multiple Choice)
4.8/5  (40)
(40)
Predict which ionic compound has the highest melting point.
(Multiple Choice)
4.9/5  (45)
(45)
A nail is coated with a 0.053 cm thick layer of zinc.The surface area of the nail is 8.59 cm2.The density of zinc is 7.13 g/cm3.How many zinc atoms are used in the coating?
(Multiple Choice)
4.9/5  (31)
(31)
Pure oxygen can exist as O2 or O3.Elements that exist in more than one distinct form are called ________.
(Short Answer)
4.8/5  (43)
(43)
Showing 61 - 80 of 105
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)