Exam 24: Carbon: Not Just Another Element
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
Which of the following is a geometric isomer of the structure given below? 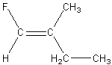
(Multiple Choice)
4.8/5  (34)
(34)
Which of the structures below has the common name m-dichlorobenzene (where m- is meta)?
(Multiple Choice)
4.9/5  (32)
(32)
How many isomers exist for the following benzene derivative,C6H4ClBr?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following statements concerning structural isomers is/are correct?
1)Structural isomers have the same elemental composition,but the atoms are linked in different ways.
2)Structural isomers have identical physical properties,but different chemical properties.
3)Structural isomers have identical structures,but contain different isotopes of the same elements.
(Multiple Choice)
4.9/5  (33)
(33)
When a secondary alcohol is oxidized,the product is a(n)____.
(Multiple Choice)
4.9/5  (30)
(30)
The molecule 2-chloro-4-methylhexane is made by addition of HCl to an alkene.Write a balanced chemical equation for this reaction.
(Essay)
4.8/5  (35)
(35)
Identify the product(s)of the hydrogenation of cis-2-hexene.
(Multiple Choice)
4.9/5  (37)
(37)
The addition of Br2 is used as the reaction to distinguish between alkanes and alkenes.What is the observation that accompanies this test?
(Multiple Choice)
4.8/5  (43)
(43)
When sulfur is added to rubber and the mixture is heated,the resulting rubber is still elastic but much stronger.This process is called
(Multiple Choice)
4.7/5  (30)
(30)
Which of the following chemical equations depicts an alkylation reaction?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following statements about polymers is/are correct?
1)Polypropylene and polyethylene are high molecular weight alkenes.
2)Addition polymers must be made from monomers which have at least one double or triple bond somewhere in the molecule.
3)Condensation polymerization reactions produce small molecules,like H2O,as byproducts to the polymer formation.
(Multiple Choice)
4.7/5  (30)
(30)
Putrescine and cadaverine are two compounds associated with decaying flesh or bad breath.These compounds belong to a class of molecules called ____.
(Multiple Choice)
4.8/5  (27)
(27)
Which one of the following hydrocarbons has cis and trans isomers?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is the correct formula for 2-methyl-1-butene?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following statements is/are CORRECT?
1)Thermoplastics are a class of polymers that will not decompose at temperatures as great as 700 C.
2)Thermosetting plastics harden when heated,but they regain their elasticity when cooled.
3)Elastomers are materials that spring back to their original shape when stretched.
(Multiple Choice)
4.9/5  (35)
(35)
Showing 41 - 60 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)