Exam 20: The Organic Chemistry of Carbohydrates
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding90 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry43 Questions
Exam 3: An Introduction to Organic Compounds: Nomenclature, physical Properties, and Structure136 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space125 Questions
Exam 5: Alkenes: Structure,nomenclature,and an Introduction to Reactivity - Thermodynamics and Kinetics84 Questions
Exam 6: The Reactions of Alkenes - the Stereochemistry of Addition Reactions89 Questions
Exam 7: The Reactions of Alkynes - Introduction to Multistep Synthesis124 Questions
Exam 8: Delocalized Electrons: Their Effect on Stability, pka, and the Products of a Reaction - Aromaticity and Electronic Effects: an Introduction to the Reactions of Benzene185 Questions
Exam 9: Substitution and Elimination Reactions of Alkyl Halides228 Questions
Exam 10: Reactions of Alcohols, ethers, epoxides, amines and Sulfur-Containing Compounds109 Questions
Exam 11: Organometallic Compounds65 Questions
Exam 12: Radicals141 Questions
Exam 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy140 Questions
Exam 14: Nmr Spectroscopy122 Questions
Exam 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives126 Questions
Exam 16: Reactions of Aldehydes and Ketones122 Questions
Exam 17: Reactions at the Α-Carbon121 Questions
Exam 18: Reactions of Benzene and Substituted Benzenes168 Questions
Exam 19: More About Amines - Reactions of Heterocylic Compounds126 Questions
Exam 20: The Organic Chemistry of Carbohydrates110 Questions
Exam 21: Amino Acids,peptides,and Proteins117 Questions
Exam 22: Catalysis in Organic Reactions and in Enzymatic Reactions92 Questions
Exam 23: The Organic Chemistry of the Coenzymes, compounds Derived From Vitamins102 Questions
Exam 24: The Organic Chemistry of the Metabolic Pathways90 Questions
Exam 25: The Organic Chemistry of Lipids37 Questions
Exam 26: The Chemistry of the Nucleic Acids94 Questions
Exam 27: Synthetic Polymers116 Questions
Exam 28: Pericyclic Reactions102 Questions
Select questions type
An optically active D-aldopentose A produced an optically active alditol B upon treatment with sodium borohydride.When this aldopentose was subjected to a Ruff degradation,a D-aldotetrose C was generated.This aldotetrose yielded an optically inactive aldaric acid D upon oxidation with nitric acid.Use these data to provide the structures of compounds A,B,C,and D.
Free
(Essay)
4.8/5  (27)
(27)
Correct Answer:
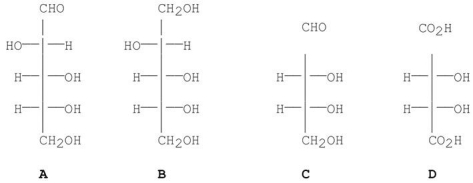
Which of the following blood types is known as the universal acceptor?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
Is the following structure of glucose a D or L configuration? Explain. 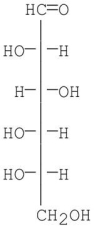
Free
(Essay)
4.9/5  (39)
(39)
Correct Answer:
This is L-glucose because the OH group attached to the bottom-most stereogenic center is on the left in the Fisher projection.
Assign the proper configurational descriptor to each asymmetric center in D-glucose below. 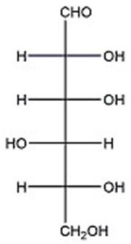
(Essay)
5.0/5  (34)
(34)
Which of the following is the cyclic hemiacetal formed from 4-hydroxyheptanal? 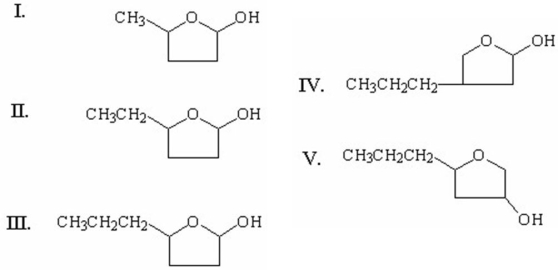
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following best represents the process of photosynthesis?
(Multiple Choice)
4.8/5  (22)
(22)
Which of the D-aldopentoses yields an optically inactive aldonic acid upon oxidation with bromine water? Explain your answer.
(Essay)
4.8/5  (30)
(30)
Which of the following best describes the sugar D-galactose? 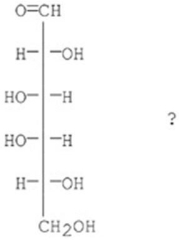
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following statements best describes the main structural difference between cellulose and chitin?
(Multiple Choice)
4.9/5  (37)
(37)
Sucrose is a disaccharide is the table sugar.It consists of
(Multiple Choice)
4.9/5  (25)
(25)
A carbohydrate composed of three to ten sugar molecules is called a(n)
(Multiple Choice)
4.8/5  (35)
(35)
Why is β-D-glucopyranose more stable in nature than α-D-glucopyranose?
(Essay)
4.8/5  (27)
(27)
Showing 1 - 20 of 110
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)