Exam 17: Temperature and the Kinetic Theory of Gases
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension103 Questions
Exam 3: Motion in Two and Three Dimensions67 Questions
Exam 4: Newtons Laws117 Questions
Exam 5: Applications of Newtons Laws75 Questions
Exam 6: Work and Energy71 Questions
Exam 7: Conservation of Energy73 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum107 Questions
Exam 9: Rotation119 Questions
Exam 10: Conservation of Angular Momentum67 Questions
Exam 11: Gravity90 Questions
Exam 12: Static Equilibrium and Elasticity65 Questions
Exam 13: Fluids91 Questions
Exam 14: Oscillations138 Questions
Exam 15: Wave Motion122 Questions
Exam 16: Superposition and Standing Waves125 Questions
Exam 17: Temperature and the Kinetic Theory of Gases85 Questions
Exam 18: Heat and the First Law of Thermodynamics114 Questions
Exam 19: The Second Law of Thermodynamics61 Questions
Exam 20: Thermal Properties and Processes54 Questions
Select questions type
A mass of He gas occupies a volume V at standard temperature and pressure. What volume is occupied if the mass is halved, the absolute temperature doubled, and the pressure increased by a third?
(Multiple Choice)
4.8/5  (45)
(45)
Calculate the point on the Fahrenheit scale when numerically it is 100 degrees larger than that of the Celsius scale for the same temperature.
(Multiple Choice)
4.8/5  (32)
(32)
What mass of He gas occupies 8.5 liters at 0 C and 1 atmosphere? (The molar mass of He = 4.00 g/mol.)
(Multiple Choice)
4.9/5  (37)
(37)
If the rms speed of oxygen molecules is 460 m/s at 0ºC, the rms speed of oxygen molecules at 273ºC is approximately
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following is NOT an assumption made in the kinetic theory model of a gas?
(Multiple Choice)
4.7/5  (33)
(33)
A 1 L container contains O2 gas at STP. If the diameter of the O2 molecule is
3.75 10 -10m, the mean free path of the O2 molecule is
(Multiple Choice)
5.0/5  (37)
(37)
Use the following to answer question: 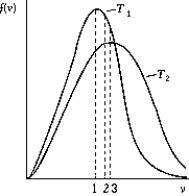 -The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
(Multiple Choice)
4.8/5  (41)
(41)
A mass of air is at pressure P and volume V at 1ºC. When it is made to occupy half this volume at three times this pressure, its temperature becomes approximately
(Multiple Choice)
4.8/5  (36)
(36)
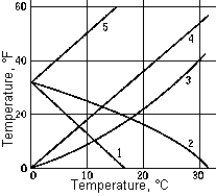 Which curve correctly represents the relation of the temperature in degrees Fahrenheit to the temperature in degrees Celsius?
Which curve correctly represents the relation of the temperature in degrees Fahrenheit to the temperature in degrees Celsius?
(Multiple Choice)
4.9/5  (30)
(30)
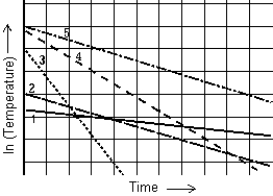 The graph shows the natural logarithm of the temperature of various thermometers as a function of time. The thermometer that cools at the quickest rate is
The graph shows the natural logarithm of the temperature of various thermometers as a function of time. The thermometer that cools at the quickest rate is
(Multiple Choice)
4.7/5  (33)
(33)
On the basis of the kinetic theory of gases, when the absolute temperature is doubled, the average kinetic energy of the gas molecules changes by a factor of
(Multiple Choice)
4.9/5  (37)
(37)
In a vacuum system, a container is pumped down to a pressure of 1.33 10-6 Pa at 20ºC. How many molecules of gas are there in 1 cm3 of this container? (Boltzmann's constant k = 1.38 10-23 J/K)
(Multiple Choice)
4.9/5  (29)
(29)
The temperature in January in Winnipeg, Manitoba, has been known to go down to -40ºC. What is this temperature on the Fahrenheit scale?
(Multiple Choice)
4.8/5  (35)
(35)
A collection of oxygen molecules occupies a volume V at standard temperature and pressure. What is the new volume if the amount of oxygen is doubled and the pressure is tripled?
(Multiple Choice)
4.8/5  (40)
(40)
Use the following to answer question:  -The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
(Multiple Choice)
4.9/5  (34)
(34)
If it is known that two bodies are in thermal equilibrium, one can conclude that
(Multiple Choice)
4.8/5  (31)
(31)
A thermometer is constructed by filling a small glass tube with a liquid that expands linearly with temperature. The thermometer is then calibrated at 0 C and 100 C, and the scale evenly divided between the two values. Unfortunately a manufacturing defect results in the middle one-third of the tube being narrower, otherwise the tube has uniform diameter. For what range of temperatures is the reading accurate? 
(Multiple Choice)
4.9/5  (39)
(39)
An ideal gas whose original temperature and volume are 27ºC and 0.283 m3 undergoes an isobaric expansion. If the final temperature is 87ºC, then the final volume is approximately
(Multiple Choice)
4.7/5  (42)
(42)
Boltzmann's constant, k, has a value of 1.381 10-23 J/K. What is the significance of the constant?
(Multiple Choice)
4.8/5  (37)
(37)
A temperature difference of 9ºF is the same as a difference of
(Multiple Choice)
4.8/5  (35)
(35)
Showing 41 - 60 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)