Exam 17: Temperature and the Kinetic Theory of Gases
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension103 Questions
Exam 3: Motion in Two and Three Dimensions67 Questions
Exam 4: Newtons Laws117 Questions
Exam 5: Applications of Newtons Laws75 Questions
Exam 6: Work and Energy71 Questions
Exam 7: Conservation of Energy73 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum107 Questions
Exam 9: Rotation119 Questions
Exam 10: Conservation of Angular Momentum67 Questions
Exam 11: Gravity90 Questions
Exam 12: Static Equilibrium and Elasticity65 Questions
Exam 13: Fluids91 Questions
Exam 14: Oscillations138 Questions
Exam 15: Wave Motion122 Questions
Exam 16: Superposition and Standing Waves125 Questions
Exam 17: Temperature and the Kinetic Theory of Gases85 Questions
Exam 18: Heat and the First Law of Thermodynamics114 Questions
Exam 19: The Second Law of Thermodynamics61 Questions
Exam 20: Thermal Properties and Processes54 Questions
Select questions type
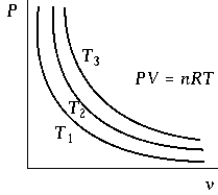 The isotherm that corresponds to the highest temperature is the one labeled
The isotherm that corresponds to the highest temperature is the one labeled
(Multiple Choice)
4.7/5  (29)
(29)
When the temperature of an ideal gas is increased from 300 K to 400 K, the average kinetic energy of the gas molecules increases by a factor of
(Multiple Choice)
4.9/5  (37)
(37)
Two monoatomic gases, helium and neon, are mixed in the ratio of 2 to 1 and are in thermal equilibrium at temperature T (the molar mass of helium = 4.0 g/mol and the molar mass of neon = 20.2 g/mol). If the average kinetic energy of each helium atom is 6.3 10-21 J, calculate the temperature T.
(Multiple Choice)
4.7/5  (33)
(33)
If it is known that two bodies are in thermal equilibrium, one can conclude that
(Multiple Choice)
4.8/5  (39)
(39)
If a mass of oxygen gas occupies a volume of 8 L at standard temperature and pressure, what is the change in the volume if the temperature is decreased to 136 K and the pressure remains constant?
(Multiple Choice)
4.9/5  (41)
(41)
The highest and lowest temperatures ever recorded in the United States are 134ºF (California, 1913) and -80ºF (Alaska, 1971). What are these temperatures in kelvins?
(Multiple Choice)
4.8/5  (32)
(32)
The temperature of the air on a hot day when the temperature is 108ºF is
(Multiple Choice)
4.8/5  (32)
(32)
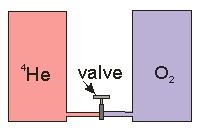 Two tanks, both of volume 10 L, are connected by a valve and are thermally isolated. One tank contains 1mole of 4He at 280 K and the other tank contains 1 mole O2 at 300 K. The valve is then opened and the gases allowed to mix. What is the final temperature after the gases have come to equilibrium?
Two tanks, both of volume 10 L, are connected by a valve and are thermally isolated. One tank contains 1mole of 4He at 280 K and the other tank contains 1 mole O2 at 300 K. The valve is then opened and the gases allowed to mix. What is the final temperature after the gases have come to equilibrium?
(Multiple Choice)
4.8/5  (38)
(38)
A device used to measure the speed, v, of molecules is shown below. When the cylinder is rotated, only molecules that are of the right speed will pass through the slanted slot and be pickup by the detector. Drive an expression for the speed v in terms of the angular speed, , , and L. L is the length of the cylinder and is the angle subtended by the slanted slot. 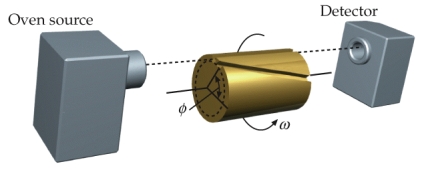
(Multiple Choice)
4.9/5  (35)
(35)
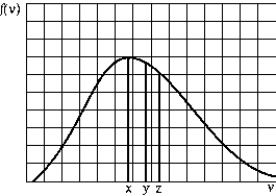 The graph shows the Maxwell-Boltzmann distribution function of the number of gas molecules per unit speed range at a given temperature. The average speed, the most probable speed, and the rms speed (in this order) are most likely given by
The graph shows the Maxwell-Boltzmann distribution function of the number of gas molecules per unit speed range at a given temperature. The average speed, the most probable speed, and the rms speed (in this order) are most likely given by
(Multiple Choice)
4.9/5  (37)
(37)
A 1 L container contains O2 gas at STP. If the diameter of the O2 molecule is 3.75 10 -10 m, the number of collisions per second is
(Multiple Choice)
4.7/5  (23)
(23)
In a Maxwell-Boltzmann distribution function of molecular speeds, the rms speed is
(Multiple Choice)
4.9/5  (40)
(40)
Doubling the Kelvin temperature of a gas increases which of the following measures of its molecular velocity by a factor of 1.4?
(Multiple Choice)
4.8/5  (38)
(38)
A constant-volume gas thermometer reads 6.66 kPa at the triple point of water. What is the pressure reading at the normal boiling point of water?
(Multiple Choice)
5.0/5  (39)
(39)
Use the following to answer question: 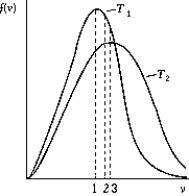 -The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
(Multiple Choice)
4.9/5  (37)
(37)
A cylinder contains oxygen gas at a temperature of 7ºC and a pressure of 15 atm in a volume of 100 L. A fitted piston is lowered into the cylinder, decreasing the volume occupied by the gas to 80 L and raising the temperature to 40ºC. The gas pressure is now approximately
(Multiple Choice)
4.9/5  (25)
(25)
A room measures 3 m 4 m 2 m and is at 15 C and 1 atm. When the temperature is increased to 25 C, the number of molecules that escaped from the room is, assuming that the pressure stays at 1 atm
(Multiple Choice)
4.9/5  (35)
(35)
A gas thermometer could measure temperature change by measuring the change in
(Multiple Choice)
4.9/5  (38)
(38)
Normal human body temperature is 98.6ºF. What is the corresponding Celsius temperature?
(Multiple Choice)
4.7/5  (37)
(37)
Showing 61 - 80 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)