Exam 17: Temperature and the Kinetic Theory of Gases
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension103 Questions
Exam 3: Motion in Two and Three Dimensions67 Questions
Exam 4: Newtons Laws117 Questions
Exam 5: Applications of Newtons Laws75 Questions
Exam 6: Work and Energy71 Questions
Exam 7: Conservation of Energy73 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum107 Questions
Exam 9: Rotation119 Questions
Exam 10: Conservation of Angular Momentum67 Questions
Exam 11: Gravity90 Questions
Exam 12: Static Equilibrium and Elasticity65 Questions
Exam 13: Fluids91 Questions
Exam 14: Oscillations138 Questions
Exam 15: Wave Motion122 Questions
Exam 16: Superposition and Standing Waves125 Questions
Exam 17: Temperature and the Kinetic Theory of Gases85 Questions
Exam 18: Heat and the First Law of Thermodynamics114 Questions
Exam 19: The Second Law of Thermodynamics61 Questions
Exam 20: Thermal Properties and Processes54 Questions
Select questions type
One mole of hydrogen gas (molar mass = 2.0 g/mol) has an rms velocity of 2.0 103 m/s. To what temperature on the Celsius scale does this correspond?
(Multiple Choice)
4.8/5  (34)
(34)
The temperature of the air on a cold day when the temperature is 23ºF is
(Multiple Choice)
5.0/5  (46)
(46)
The air in a balloon occupies a volume of 0.10 m3 when at a temperature of 27ºC and a pressure of 1.2 atm. What is the balloon's volume at 7ºC and 1.0 atm? (The amount of gas remains constant.)
(Multiple Choice)
4.8/5  (38)
(38)
You can double both the pressure and the volume of an ideal gas if you change the temperature of the gas by
(Multiple Choice)
5.0/5  (42)
(42)
Which of the following statements about absolute zero temperature is true?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following statements about the ideal gas law, PV = nRT, is NOT true?
(Multiple Choice)
4.8/5  (35)
(35)
The relationship between the pressure and the volume of a gas expressed by Boyle's law holds true
(Multiple Choice)
4.9/5  (44)
(44)
If both the temperature and the volume of an ideal gas are doubled, the pressure is
(Multiple Choice)
5.0/5  (35)
(35)
Inside a sphere of radius 12 cm are 8.0 1023 gas molecules at a temperature of 50 C. What pressure do the gas molecules exert on the inside of the sphere?
(Multiple Choice)
4.8/5  (36)
(36)
The temperature at which the Celsius and Fahrenheit scales read the same is
(Multiple Choice)
4.8/5  (35)
(35)
At what common Celsius temperature is the rms velocity of oxygen molecules (molar mass = 32 g/mol) double that of hydrogen molecules (molar mass = 2.0 g/mol)?
(Multiple Choice)
4.8/5  (34)
(34)
A room measures 3 m 4 m 2 m and is at 20 C and 1 atm. Assuming that it only has the two diatomic gases, N2 and O2, the amount of kinetic energy in the gases is
(Multiple Choice)
4.9/5  (38)
(38)
The air around us has 78% nitrogen and 21% oxygen. If the pressure is 1 atm, the pressure due to oxygen is
(Multiple Choice)
4.7/5  (35)
(35)
A gas has a density X at standard temperature and pressure. What is the new density when the absolute temperature is doubled and the pressure increased by a factor of 3?
(Multiple Choice)
4.8/5  (36)
(36)
If the absolute temperature of a gas is doubled, what is the change in the rms speed of its molecules?
(Multiple Choice)
4.7/5  (35)
(35)
Five molecules of a gas have the following speeds:
200 m/s,
300 m/s,
400 m/s,
500 m/s, and
600 m/s.
The rms speed for these molecules is
(Multiple Choice)
4.9/5  (39)
(39)
If the absolute temperature of a gas is doubled, what is the change in the average kinetic energy of its molecules?
(Multiple Choice)
4.8/5  (38)
(38)
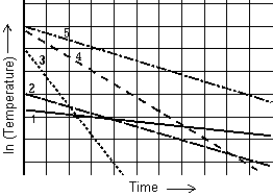 The graph shows the natural logarithm of the temperature of various thermometers as a function of time. The thermometer that cools at the slowest rate is
The graph shows the natural logarithm of the temperature of various thermometers as a function of time. The thermometer that cools at the slowest rate is
(Multiple Choice)
4.8/5  (35)
(35)
At room temperature, which of the following diatomic molecules has the greater average kinetic energy: carbon monoxide (molar mass = 28 g/mol), nitrogen (molar mass = 28 g/mol), or oxygen (molar mass = 32 g/mol)?
(Multiple Choice)
4.9/5  (37)
(37)
If a mass of oxygen gas occupies a volume of 8 L at standard temperature and pressure, what is the change in the volume if the temperature is reduced by one half and the pressure is doubled?
(Multiple Choice)
4.8/5  (44)
(44)
Showing 21 - 40 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)