Exam 17: Temperature and the Kinetic Theory of Gases
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension103 Questions
Exam 3: Motion in Two and Three Dimensions67 Questions
Exam 4: Newtons Laws117 Questions
Exam 5: Applications of Newtons Laws75 Questions
Exam 6: Work and Energy71 Questions
Exam 7: Conservation of Energy73 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum107 Questions
Exam 9: Rotation119 Questions
Exam 10: Conservation of Angular Momentum67 Questions
Exam 11: Gravity90 Questions
Exam 12: Static Equilibrium and Elasticity65 Questions
Exam 13: Fluids91 Questions
Exam 14: Oscillations138 Questions
Exam 15: Wave Motion122 Questions
Exam 16: Superposition and Standing Waves125 Questions
Exam 17: Temperature and the Kinetic Theory of Gases85 Questions
Exam 18: Heat and the First Law of Thermodynamics114 Questions
Exam 19: The Second Law of Thermodynamics61 Questions
Exam 20: Thermal Properties and Processes54 Questions
Select questions type
The oxygen (molar mass = 32 g/mol) and nitrogen (molar mass = 28 g/mol) molecules in this room have equal average
(Multiple Choice)
4.9/5  (49)
(49)
Two cylinders are connected by a small tube which has a needle valve in the middle. One cylinder contains 4He gas, and the other cylinder contains CO2 gas. The gases are at the same temperature, pressure and volume. The lids that seal the gases in are free to move up or down. When the valve is opened, what happens to the volume in each cylinder? 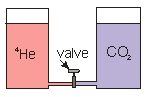
(Multiple Choice)
4.8/5  (37)
(37)
A temperature difference of 5 Cº is the same as a difference of
(Multiple Choice)
4.8/5  (39)
(39)
If you carry a sealed cylinder of oxygen to the moon, you should expect no change in the oxygen's
(Multiple Choice)
4.7/5  (37)
(37)
Use the following to answer question: 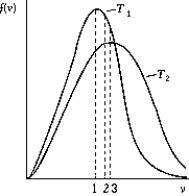 -The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
(Multiple Choice)
4.9/5  (37)
(37)
Showing 81 - 85 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)