Exam 21: Transition Metals and Coordination Chemistry
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
How many of the following compounds exhibit geometric isomers?
I.Pt(NH3)2Cl2 (square planar)
II.[Co(H2O)2]Cl3
III.Ni(NH3)4(NO2)2
IV.K2[CoCl4]
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is true about coordination complexes?
(Multiple Choice)
4.7/5  (28)
(28)
The 3d electrons in Co(NH3)63+ are all paired but Fe(H2O)63+ has unpaired electrons (is paramagnetic).Explain.
(Essay)
4.9/5  (42)
(42)
In the crystal field model,ligands are treated as negative point charges.
(True/False)
4.9/5  (26)
(26)
Here are some crystal field representations of d electrons in an octahedral complex:
I) 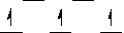 II)
II) 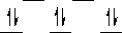 III)
III) 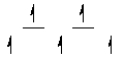 IV)
IV) 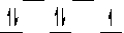 V)
V) 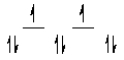 Choose the representation that fits the transition metal atom in the following species:
-Fe(OH2)63+ (assume weak field)
Choose the representation that fits the transition metal atom in the following species:
-Fe(OH2)63+ (assume weak field)
(Multiple Choice)
4.8/5  (35)
(35)
The empirical formula of a compound with a mass percent composition of 6.78% H,31.43% N,39.76% Cl,and 22.03% Co is consistent with which of the following complexes?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the transition metals is the best conductor of heat and electric current?
(Multiple Choice)
4.8/5  (40)
(40)
A coordination compound of Cu2+ can be described as Cu(NH3)xSO4 and is known to contain 29.92% NH3 by mass.The value of x is:
(Multiple Choice)
4.9/5  (35)
(35)
The metals with the highest ionization energies are most likely to be found in nature in the elemental state.
(True/False)
4.8/5  (33)
(33)
In which of the following complexes does the transition metal have a d8 configuration?
(Multiple Choice)
4.9/5  (51)
(51)
For the process Co(NH3)5Cl2+ + Cl- Co(NH3)4Cl2+ + NH3 what would be the ratio of cis to trans isomer in the product?
(Multiple Choice)
5.0/5  (39)
(39)
Oxygen is stored in mammalian tissue in which type of molecule?
(Multiple Choice)
4.7/5  (35)
(35)
How many unpaired electrons are found in each of the following complex ions?
-[Cr(CN)4]2-
(Short Answer)
4.8/5  (41)
(41)
Showing 41 - 60 of 142
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)