Exam 21: Transition Metals and Coordination Chemistry
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
Which transition metal is valued for its high electrical conductivity and resistance to corrosion,and is widely used for plumbing?
(Multiple Choice)
4.8/5  (41)
(41)
How many unpaired electrons are there in the tetrahedral complex ion [FeCl4]-?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following are structural isomers?
I.coordination isomers
II.linkage isomers
III.geometrical isomers
IV.optical isomers
(Multiple Choice)
4.8/5  (37)
(37)
The complex FeL62+,where L is a neutral ligand,is known to be diamagnetic.The number of d electrons in this complex ion is:
(Multiple Choice)
4.8/5  (40)
(40)
What are the oxidation numbers of each central metal atom in the following coordination compounds? K3[Fe(CN)6],[Cr(NH3)4Br2]Br,[Ni(H2O)6]Cl2,Na2[TaF7]
(Multiple Choice)
5.0/5  (39)
(39)
Which of the following ligands are capable of linkage isomerism?
N-
NO2-
NH3
NH2CH2CH2NH2
OCN-
Cl-
H2O
SCN-
(Essay)
4.8/5  (36)
(36)
Here are some crystal field representations of d electrons in an octahedral complex:
I) 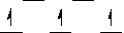 II)
II) 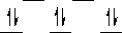 III)
III) 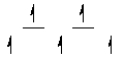 IV)
IV) 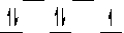 V)
V) 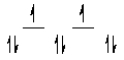 Choose the representation that fits the transition metal atom in the following species:
-K4Mn(CN)6 (assume strong field)
Choose the representation that fits the transition metal atom in the following species:
-K4Mn(CN)6 (assume strong field)
(Multiple Choice)
4.8/5  (40)
(40)
How many d electrons are present on the metal ion in the complex ion PtCl62-?
(Multiple Choice)
4.8/5  (35)
(35)
Addition of AgNO3 to aqueous solutions of the complex results in a cloudy white precipitate,presumably AgCl.You dissolve 0.1000 g of the complex in H2O and perform a precipitation titration with 0.0500 M AgNO3 as the titrant.Using an electrode that is sensitive to [Ag+],you reach the endpoint after 9.00 mL of titrant are added.How many grams of chloride ion were present in the 0.1000-g sample?
(Multiple Choice)
4.8/5  (41)
(41)
The spectrochemical series is I- < Br- < Cl- < F- < OH- < H2O < NH3 < en < NO2- < CN- Which of the following complexes will absorb visible radiation of the highest energy (shortest wavelength)?
(Multiple Choice)
4.8/5  (40)
(40)
A certain complex ion has a distorted octahedral structure in which the ligands along the plus and minus z axes are compressed (pushed in closer to the central metal ion).The d orbital splitting diagram for this complex ion would be:
(Multiple Choice)
4.7/5  (41)
(41)
What is the formula for the pentaamminehydroxocopper(II)ion?
(Multiple Choice)
4.9/5  (36)
(36)
The complex ion Ni(NH3)62+ (two unpaired electrons)is classified as:
(Multiple Choice)
4.8/5  (34)
(34)
The reducing abilities of the first-row transition metals generally __________ going from left to right across the period.
(Multiple Choice)
4.8/5  (36)
(36)
Showing 61 - 80 of 142
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)