Exam 21: Transition Metals and Coordination Chemistry
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
The phenomenon called __________ contraction is responsible for the great similarity in atomic size and chemistry of 4d and 5d elements.
(Multiple Choice)
4.9/5  (36)
(36)
Which metal is most widely used in the electrical systems of homes and factories?
(Multiple Choice)
4.8/5  (41)
(41)
When 6.3 moles of [Co(NH3)5Cl]Cl2 is dissolved in water,how many moles of ions are in solution?
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following complexes can exhibit optical isomerism? (en = H2N-CH2-CH2-NH2 and is bidentate)
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following transition metals are important to the U.S.economy and defense?
(Multiple Choice)
4.7/5  (36)
(36)
Calculate the total number of unpaired electrons in the following complex ions: Zn(OH2)62+,Ni(CN)42- (square planar),Co(NH3)63+ (strong field).
(Multiple Choice)
4.9/5  (45)
(45)
How many unpaired electrons are there in Ir(Br)64-? (Br- is a weak-field ligand. )
(Multiple Choice)
4.9/5  (43)
(43)
The complex ions of Zn2+ are all colorless.The most likely explanation for this is:
(Multiple Choice)
4.8/5  (40)
(40)
What transition metal has the combination of toughness,stretchability,and resilience that makes it ideal for use in bicycle frames?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following crystal field diagrams is correct for Mn(CN)63- (CN- is a strong field ligand)?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following crystal field diagrams is correct for Co(CN)64- where CN- is a strong field ligand?
(Multiple Choice)
4.9/5  (41)
(41)
How many unpaired electrons are there in the complex ion [Co(NO3)6]4-? For this ion the nitrate ligands produce a very strong crystal field.
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements is true about the octahedral complexes of Ni2+?
(Multiple Choice)
4.8/5  (38)
(38)
How many unpaired electrons are found in each of the following complex ions?
-FeCl42-
(Short Answer)
4.9/5  (32)
(32)
The complex ion NiCl42- is tetrahedral.The number of unpaired electrons in the complex is:
(Multiple Choice)
4.9/5  (41)
(41)
Consider the pseudo-octahedral complex of Cr3+ shown below,where A and B represent Lewis bases and where A produces a stronger crystal field than B.Draw an appropriate crystal field diagram for this complex (include the electrons). 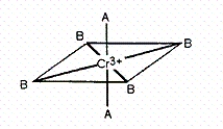
(Essay)
4.8/5  (31)
(31)
Showing 81 - 100 of 142
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)