Exam 21: Transition Metals and Coordination Chemistry
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
Here are some crystal field representations of d electrons in an octahedral complex:
I) ![Here are some crystal field representations of d electrons in an octahedral complex: I) II) III) IV) V) Choose the representation that fits the transition metal atom in the following species: -[Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup>](https://storage.examlex.com/TB6423/11eaa8f0_66c1_ddf6_93a6_732a54800862_TB6423_00_TB6423_00_TB6423_00_TB6423_00_TB6423_00.jpg) II)
II) ![Here are some crystal field representations of d electrons in an octahedral complex: I) II) III) IV) V) Choose the representation that fits the transition metal atom in the following species: -[Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup>](https://storage.examlex.com/TB6423/11eaa8f0_66c1_ddf7_93a6_c76c4439e0b7_TB6423_00_TB6423_00_TB6423_00_TB6423_00_TB6423_00.jpg) III)
III) ![Here are some crystal field representations of d electrons in an octahedral complex: I) II) III) IV) V) Choose the representation that fits the transition metal atom in the following species: -[Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup>](https://storage.examlex.com/TB6423/11eaa8f0_66c2_0508_93a6_615952f13786_TB6423_00_TB6423_00_TB6423_00_TB6423_00_TB6423_00.jpg) IV)
IV) ![Here are some crystal field representations of d electrons in an octahedral complex: I) II) III) IV) V) Choose the representation that fits the transition metal atom in the following species: -[Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup>](https://storage.examlex.com/TB6423/11eaa8f0_66c2_0509_93a6_25825bed7e96_TB6423_00_TB6423_00_TB6423_00_TB6423_00_TB6423_00.jpg) V)
V) ![Here are some crystal field representations of d electrons in an octahedral complex: I) II) III) IV) V) Choose the representation that fits the transition metal atom in the following species: -[Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup>](https://storage.examlex.com/TB6423/11eaa8f0_66c2_050a_93a6_0f3172436323_TB6423_00_TB6423_00_TB6423_00_TB6423_00_TB6423_00.jpg) Choose the representation that fits the transition metal atom in the following species:
-[Co(NH3)4Br2]+
Choose the representation that fits the transition metal atom in the following species:
-[Co(NH3)4Br2]+
(Multiple Choice)
4.8/5  (35)
(35)
The strength of steel is due to the effect of what substance with the iron?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following is not a typical step in metallurgy?
(Multiple Choice)
4.9/5  (40)
(40)
What heavy metal is the most abundant and most important to our civilization?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following is incorrect concerning the 3d,4d,and 5d transition series?
(Multiple Choice)
4.8/5  (43)
(43)
The geometry of a coordination compound with a coordination number of 4 is
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following is true in describing the crystal field model?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following complexes shows geometrical isomerism?
(Multiple Choice)
4.9/5  (39)
(39)
How many ligands are in this representative drawing of a complex ion? 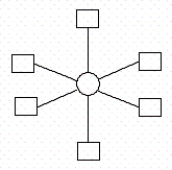
(Multiple Choice)
4.8/5  (33)
(33)
A d6 ion (Fe2+)is complexed with six strong-field ligands (for example,SCN-).What is the number of unpaired electrons in this complex?
(Multiple Choice)
4.7/5  (46)
(46)
Give the number of geometrical isomers for the octahedral compound [MA2B2C2],where A,B,and C represent ligands.
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following coordination compounds will form a precipitate when treated with an aqueous solution of AgNO3?
(Multiple Choice)
4.9/5  (32)
(32)
Suppose you are studying coordination compounds of Co(II)with the ligand pyridine (py,C5H5N,molar mass = 79.10).You isolate a crystalline compound,and since the only available anions are Cl- and NO3-,you hypothesize the empirical formula of the coordination compound must be Cow(py)x(Cl)y(NO3)z.
-You discover that the complex decomposes in water.You dissolve 0.1000 g of the complex in H2O and add excess NaHg(SCN)4,which precipitates Co(II)as CoHg(SCN)4(s).After the precipitate is washed and dried,its mass is 0.1102 g.How many grams of cobalt are contained in 0.1000 g of the complex?
(Multiple Choice)
4.9/5  (39)
(39)
How many unpaired electrons are found in each of the following complex ions?
-Fe(CN)64-
(Short Answer)
4.8/5  (38)
(38)
The ____ isomer of the complex Ni(en)2Cl2 exhibits optical isomers,but the _____ isomer does not.
(Multiple Choice)
4.8/5  (29)
(29)
Showing 121 - 140 of 142
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)