Exam 10: The Humoral Immune Response
Exam 1: Basic Concepts in Immunology44 Questions
Exam 2: Innate Immunity: the First Lines of Defense32 Questions
Exam 3: The Induced Responses of Innate Immunity39 Questions
Exam 4: Antigen Recognition by B-Cell and T-Cell Receptors28 Questions
Exam 5: The Generation of Lymphocyte Antigen Receptors33 Questions
Exam 6: Antigen Presentation to T Lymphocytes30 Questions
Exam 7: Lymphocyte Receptor Signaling42 Questions
Exam 8: Development and Survival of Lymphocytes37 Questions
Exam 9: T-Cell-Mediated Immunity37 Questions
Exam 10: The Humoral Immune Response30 Questions
Exam 11: Integrated Dynamics of Innate and Adaptive Immunity28 Questions
Exam 12: The Mucosal Immune System27 Questions
Exam 13: Failures of Host Defense Mechanisms43 Questions
Exam 14: Allergy and Allergic Diseases26 Questions
Exam 15: Autoimmunity and Transplantation31 Questions
Exam 16: Manipulation of the Immune Response34 Questions
Select questions type
The germinal center is a region within the secondary B cell follicle where sustained B cell proliferation and differentiation take place. The processes of B cell proliferation and differentiation, including affinity maturation and class switching, require periodic interactions of the germinal center B cells with CD4 TFH cells. These periodic interactions between the B cells and TFH cells can occur:
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
A
In cell culture experiments, purified B cells expressing IgM can be induced to switch to producing IgE by stimulating them with an antibody to CD40 (a stimulatory antibody) plus the cytokine IL-4. In an individual undergoing an immune response, these signals would normally be provided by:
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
E
Surprisingly, individuals with defects in the early components of the classical complement cascade suffer from an autoimmune type of kidney damage, rather than from an immunodeficiency leading to increased susceptibility to infections. Why do these complement defects lead to autoimmune kidney damage?
Free
(Essay)
4.7/5  (27)
(27)
Correct Answer:
Complement deposition on immune complexes is important in the clearance from the circulation. In the absence of this mechanism, immune complexes accumulate in the circulation and get deposited in the basement membranes of small blood vessels, most notably those of the renal glomerulus, where the blood is filtered to form urine. Immune complexes then cause kidney damage.
Some individuals with repetitive exposure to high doses of Schistosoma mansoni develop resistance to re-infection by this helminthic parasite. In contrast, other individuals remain highly susceptible. Population studies showed that resistant individuals had increased numbers of circulating eosinophils in their blood compared to susceptible individuals, and further, that these eosinophils had increased levels of:
(Multiple Choice)
4.7/5  (35)
(35)
Individuals with a genetic polymorphism in the Fc receptor, Fc RIIa (CD32), have an increased susceptibility to bacterial meningitis (inflammation of the membranes (meninges) surrounding the brain and spinal cord) caused by the encapsulated bacterium, Neisseria meningitidis. This polymorphism reduces the efficiency with which the phagocytes expressing Fc RIIa bind to the constant region of this receptor's target antibody. The reason this Fc RIIa-dependent response it the major form of protection against Neisseria meningitidis is because:
(Multiple Choice)
4.8/5  (37)
(37)
When vesicular stomatitis virus (VSV) is used to infect mice via footpad injection, viral particles are trapped in the draining lymph node (the popliteal lymph node) within 5 minutes of injection. These viral particles are then retained in the lymph node for many hours, where they can be visualized on cells that are interacting with B cells. The cells retaining the viral particles in the lymph node are not tissue-resident dendritic cells that have migrated to the lymph node with the virus, as this process takes much longer than 5 minutes. In which region of the lymph node would you expect to find the trapped viral particles and on which cells?
(Essay)
4.9/5  (37)
(37)
CXCR5 is the receptor for the chemokine CXCL13, secreted by follicular stromal cells and follicular dendritic cells in the B cell zones (i.e., lymphoid follicles) of secondary lymphoid organs. A conditional knockout mouse in which CXCR5 was specifically deleted only in T cells would have:
(Multiple Choice)
4.9/5  (43)
(43)
Individuals infected with herpes simplex virus (HSV) mount protective antibody responses directed against the surface glycoproteins of the virus. These antibodies are critical to viral clearance, contributing to viral neutralization as well as to complement-mediated and cytotoxic cell-mediated killing of infected target cells. Surprisingly, humans as well as mice deficient in the complement protein, C3, have greatly reduced antibody responses to T cell-dependent antigens, and are impaired in their ability to control HSV infections. When C3-deficient mice are infected with HSV, once at day 0 and then a second time 4 weeks later, their antibody response is altered compared to wild-type mice, as shown in Figure. 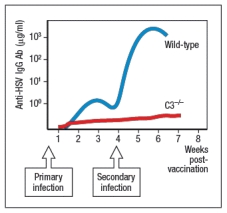 a) What is a likely explanation for the altered antibody response in the absence of complement C3?
To test your hypothesis, the following experiment is performed. Wild-type and C3-/- mice are each inoculated with a replication-defective form of HSV (HSV-rd). This virus infects cells, stimulates innate immune responses, but is not able to replicate and spread in the body. For this experiment, mice are infected with varying doses of the HSV-rd virus, and peak IgG responses to the viral surface glycoproteins are measured. The results are shown in Figure.
a) What is a likely explanation for the altered antibody response in the absence of complement C3?
To test your hypothesis, the following experiment is performed. Wild-type and C3-/- mice are each inoculated with a replication-defective form of HSV (HSV-rd). This virus infects cells, stimulates innate immune responses, but is not able to replicate and spread in the body. For this experiment, mice are infected with varying doses of the HSV-rd virus, and peak IgG responses to the viral surface glycoproteins are measured. The results are shown in Figure. 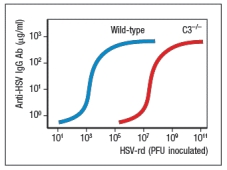 b) What is the most likely explanation for these data? Do these results impact your answer to part (a)? Explain your reasoning.
To further elucidate the function of complement C3 in the humoral immune response, a knockout mouse line lacking the receptor for C3 fragments is generated. This receptor is encoded by the Cr2 gene, so knockout mice lacking this gene are known as Cr2-/-.
Previous studies have indicated that the complement receptor encoded by the Cr2 gene is expressed on B cells and on the follicular dendritic cells that reside in B cell zones of secondary lymphoid organs. To distinguish the functions of the complement receptor on these two cell types, a series of bone marrow chimeras are generated. Wild-type bone marrow is used to reconstitute lethally irradiated Cr2-/- mice (wt Cr2-/-), or vice versa (Cr2-/- wt). As controls, Cr2-/- bone marrow is used to reconstitute Cr2-/- recipients (Cr2-/- Cr2-/-), and wild-type bone marrow used to reconstitute wild-type recipients (wt wt). These mice are then inoculated with the HSV-rd virus at 106 PFU, once at day 0 and then a second time at day 28, and the anti-HSV IgG responses are measured every 7 days, as shown in Figure.
b) What is the most likely explanation for these data? Do these results impact your answer to part (a)? Explain your reasoning.
To further elucidate the function of complement C3 in the humoral immune response, a knockout mouse line lacking the receptor for C3 fragments is generated. This receptor is encoded by the Cr2 gene, so knockout mice lacking this gene are known as Cr2-/-.
Previous studies have indicated that the complement receptor encoded by the Cr2 gene is expressed on B cells and on the follicular dendritic cells that reside in B cell zones of secondary lymphoid organs. To distinguish the functions of the complement receptor on these two cell types, a series of bone marrow chimeras are generated. Wild-type bone marrow is used to reconstitute lethally irradiated Cr2-/- mice (wt Cr2-/-), or vice versa (Cr2-/- wt). As controls, Cr2-/- bone marrow is used to reconstitute Cr2-/- recipients (Cr2-/- Cr2-/-), and wild-type bone marrow used to reconstitute wild-type recipients (wt wt). These mice are then inoculated with the HSV-rd virus at 106 PFU, once at day 0 and then a second time at day 28, and the anti-HSV IgG responses are measured every 7 days, as shown in Figure. 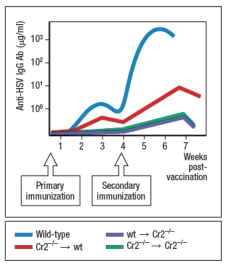 c) From the data shown above, on which cell type is the expression of the complement receptor most important for humoral immunity?
d) For each of the cell types expressing the complement receptor encoded by Cr2, what is the explanation for their importance in humoral immunity to HSV inoculation?
c) From the data shown above, on which cell type is the expression of the complement receptor most important for humoral immunity?
d) For each of the cell types expressing the complement receptor encoded by Cr2, what is the explanation for their importance in humoral immunity to HSV inoculation?
(Essay)
4.9/5  (40)
(40)
Patients with the disease X-linked lymphoproliferative syndrome (XLP) lack expression of the small adapter protein SAP, which associates with receptors of the SLAM family. One characteristic of this disease is an inability of cytotoxic T cells to control infections with a virus, Epstein-Barr virus (EBV), that replicates in B cells. This defect in control of EBV results from:
(Multiple Choice)
4.9/5  (46)
(46)
Mice and humans with inactivating mutations in the gene encoding activation-induced cytidine deaminase (AID) have an immunodeficiency disease known as 'hyper IgM type 2'. Since AID is the enzyme that catalyzes the conversion of cytosines in the DNA to uracils, thereby initiating the process of somatic hypermutation, why do individuals with this deficiency only produce IgM antibodies?
(Essay)
4.7/5  (36)
(36)
The upper respiratory tract of many individuals is colonized by Streptococcus pneumoniae bacteria. Infections caused by these bacteria, including pneumonia, meningitis, otitis media (ear infections), and sinusitis, are thought to occur in individuals lacking protective antibodies. For many years, IgG was thought to be the major antibody class responsible for protective immunity to S. pneumoniae, due to the ability of anti-bacterial capsule IgG antibodies to opsonize the bacteria and promote phagocytosis. However, in addition to IgG, pneumococcal polysaccharides elicit robust IgA antibody responses. It was traditionally thought that these IgA antibodies functioned in neutralization, by blocking bacterial attachment to mucosal epithelial cells. It is now known that IgA antibodies, like IgG, can function as opsonins, to induce phagocytosis and killing of IgA-coated pathogens. This function of IgA antibodies depends on:
(Multiple Choice)
4.7/5  (34)
(34)
Irradiation of mice with a dose of 600 rad of total body irradiation eliminates 95% of total lymphocytes from spleen and lymph nodes and also eliminates all antigen-specific memory B cells. Nonetheless, when Influenza A-infected mice are subjected to this irradiation at 60-days post-infection, and then reconstituted with bone marrow cells from a naive mouse (this replenishes all of the lymphocyte populations), the levels of circulating anti-Influenza A IgG antibodies show nearly no decline when mice are monitored for the following year. What is the explanation for this finding?
(Essay)
4.7/5  (33)
(33)
IgM antibodies are much more efficient than IgG at activating the complement cascade. However, under certain circumstances, IgG antibodies will activate the complement pathway. One example of a situation in which IgG binding to its antigen will not trigger the complement cascade is when:
(Multiple Choice)
4.8/5  (40)
(40)
Once B cells begin secreting antibodies, they cease dividing and have a life-span of only a few days.
(True/False)
4.8/5  (36)
(36)
Wild-type mice infected with one strain of Influenza A virus (PR8) by intranasal inoculation are protected from intranasal infection by a related Influenza A virus (Beijing), a phenomenon known as cross-protection. These infections are generally localized to the upper respiratory tract. Mice with a homozygous single gene defect in 'gene X' have greatly impaired cross-protection to Influenza A-Beijing following immunization with Influenza A-PR8 by the intranasal route. Gene X likely encodes:
(Multiple Choice)
4.9/5  (40)
(40)
Unlike somatic hypermutation, class switching occurs in discrete sequence regions upstream of the immunoglobulin heavy chain coding sequences (called switch regions). One key element in directing the enzyme AID to a specific switch region is the opening of the DNA duplex combined with polymerase stalling during active transcription in that region. A second key feature of directing AID to a specific switch region is:
(Multiple Choice)
4.8/5  (35)
(35)
Individuals with allergies often mount IgE antibody responses to non-infectious environmental antigens. Examples are peanut allergies, allergies to house dust mites, and allergic responses to bee stings. In all of these cases, the allergic response is mediated by the cross-linking of the high affinity IgE receptors on mast cells (Fc RI) which are pre-bound to the allergen-specific IgE antibodies. Yet, the symptoms of these different allergic responses can vary greatly, and can include skin rashes or hives, swelling of the throat and difficulty breathing, to gastrointestinal symptoms such as cramps, diarrhea or vomiting. How can different IgE-mediated allergic responses cause such different symptoms?
(Essay)
4.9/5  (43)
(43)
In humans, IgA is produced in copious amounts, estimated to be a rate of 3 g/day. Nearly all of the IgA secreting plasma cells are found in the gastrointestinal (GI) tract where the secreted IgA is transported across the GI epithelium into the lumen of the gut. There, this antibody protects the GI epithelium against intestinal pathogens. In contrast, none of the GI resident long-lived antibody secreting cells produce antibodies of the IgG class. The differential localization of long-lived antibody secreting cells producing IgA compared to those producing IgG is likely due to:
(Multiple Choice)
4.8/5  (37)
(37)
Borrelia hermsii is a spirochete bacterium, transmitted by tick bites, that causes an illness characterized by a relapsing fever. The bacteria enter the host bloodstream and replicate there. Studies in mice show that episodes of bacteremia (bacteria in the blood) are efficiently controlled by anti-bacterial antibodies, but interestingly, follicular B cells are not required for this response, nor is the response impaired by splenectomizing the mice (i.e., removing the spleen). Which B cells are most likely responsible for this antibody response?
(Essay)
4.9/5  (31)
(31)
Antibody-dependent cell-mediated cytotoxicity (ADCC) is an important effector mechanism in immunity to virus infections. This immune pathway has also been exploited for clinical applications. For instance, patients with various disorders, including rheumatoid arthritis and some B cell lymphomas, are treated with an antibody directed at CD20, a surface receptor expressed on all B cells. This antibody leads to the depletion of B cells from the patients by the actions of:
(Multiple Choice)
4.8/5  (40)
(40)
Showing 1 - 20 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)