Exam 7: Lymphocyte Receptor Signaling
Several small GTPases play critical roles in antigen receptor signaling pathways. When activated by binding to GTP, these mediators induce changes in cytoskeletal organization, adhesion, and metabolism, but have no role in transcription factor activation.
False
Antigen receptor signaling pathways are regulated by a balanced equilibrium between tyrosine kinases and tyrosine phosphatases. In general, activation of signaling proceeds when the kinase activities leading to auto-phosphorylation of Lck, to phosphorylation of ZAP-70, and to phosphorylation of downstream adapters and scaffolds exceeds the activity of phosphatases acting on these substrates. Therefore, it came as a surprise when T cells lacking the membrane tyrosine phosphatase, CD45, were first generated, and were found to be unable to be activated by TCR stimulation. Name one important function of CD45 in T cells that explains the requirement for this phosphatase in TCR signaling.
CD45 is the phosphatase that de-phosphorylates the C-terminal negative regulatory tyrosine of Lck. When this negative regulatory tyrosine is phosphorylated and binds to the Lck SH2 domain, Lck is held in an inactive conformation. TCR signaling initiated by Lck cannot occur without CD45 to dephosphorylate this site.
Antigen receptor signaling and lymphocyte activation.antibody coupled to biotin, followed by cross-linking with Streptavidin (S-Av). As the antibody and then S-Av are added, the cells are run on the flow cytometer to examine the fluorescence of the Ca2+-sensitive dye. After several minutes of analysis, the cells are stimulated with ionomycin (Iono), to induce Ca2+ influx; this is used as a positive control to ensure that the cells are loaded with the dye. In Figure Q41)A, the characteristic pattern of Ca2+ influx is shown in the red line (wild-type; WT), where TCR stimulation causes a sharp rise in cytoplasmic Ca2+, followed by a slow decline over hours. As shown below, cytoplasmic Ca2+ concentrations do not normally return to baseline for the timecourse of this experiment. A mutant mouse is identified with a defect in T cell activation in response to TCR stimulation. The calcium response of T cells from the mutant mouse is shown in the blue line.  a) Given these data, name three T cell signaling proteins that could be defective in the mutant T cells. Then name three T cell signaling proteins that could not be responsible for this defect, even if mutated.
Additional experiments are performed to analyze protein tyrosine phosphorylation in response to TCR stimulation. For these experiments, T cells are stimulated with anti-CD3 antibody, and then lysates are prepared and run on a protein (SDS-PAGE) gel to separate the proteins by molecular weight. The proteins are transferred from the gel to a membrane for immunoblotting using an antibody that binds to all phosphorylated tyrosine residues in any protein; this antibody is called ‘anti-phospho-tyrosine antibody,’ and is abbreviated as anti-P-Y. The results are shown in Figure .
a) Given these data, name three T cell signaling proteins that could be defective in the mutant T cells. Then name three T cell signaling proteins that could not be responsible for this defect, even if mutated.
Additional experiments are performed to analyze protein tyrosine phosphorylation in response to TCR stimulation. For these experiments, T cells are stimulated with anti-CD3 antibody, and then lysates are prepared and run on a protein (SDS-PAGE) gel to separate the proteins by molecular weight. The proteins are transferred from the gel to a membrane for immunoblotting using an antibody that binds to all phosphorylated tyrosine residues in any protein; this antibody is called ‘anti-phospho-tyrosine antibody,’ and is abbreviated as anti-P-Y. The results are shown in Figure .
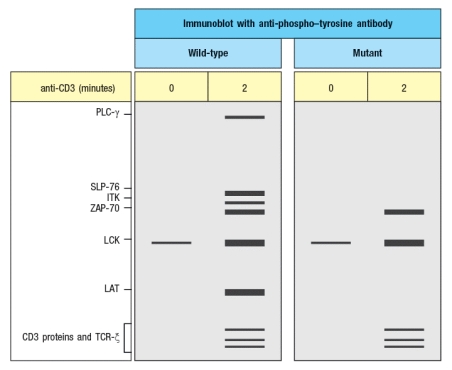 You confirm that the mutant T cells express normal levels of all the proteins detected in the WT cells, including PLC- , SLP-76, ITK, ZAP-70, LCK, LAT, and the CD3 and TCR proteins.
b) Based on these additional data, which of the candidate proteins in your answer to part (a) are ruled out? Briefly explain your answer.
c) What protein is most likely defective in the mutant cells and why?
d) For the protein you named in your answer to part (c), which amino acids or domain of the protein could be mutated to account for all the data.
You confirm that the mutant T cells express normal levels of all the proteins detected in the WT cells, including PLC- , SLP-76, ITK, ZAP-70, LCK, LAT, and the CD3 and TCR proteins.
b) Based on these additional data, which of the candidate proteins in your answer to part (a) are ruled out? Briefly explain your answer.
c) What protein is most likely defective in the mutant cells and why?
d) For the protein you named in your answer to part (c), which amino acids or domain of the protein could be mutated to account for all the data.
a) Any TCR signaling protein required for PLC- activation could be responsible. This would include Lck, ZAP-70, SLP-76, LAT, ITK, or PLC- itself. Additional candidates could be PI 3-kinase, ORAI1, or STIM1. Proteins that could not be responsible for the calcium signaling defect include: WASp, cdc42, Vav, Akt, mTOR, Rheb, Protein kinase C- , Rap1, Nck, Ras, RasGRP, Erk-MAP-kinase or calcineurin. This is not a complete list, but the most common .
b) LCK is normal, as CD3 and TCR phosphorylation are normal. Also, increased LCK phosphorylation in response to TCR stimulation (autophosphorylation) is also normal. In addition, ZAP-70 phosphorylation is normal, indicating normal LCK activity. Since there is no phosphorylation of LAT or SLP-76, there is likely a defect in ZAP-70 kinase activity. These data cannot directly rule out a defect in ITK or PLC- , but given the lack of phosphorylation of SLP-76 and LAT, a more likely explanation is a defect in ZAP-70.
c) ZAP-70 for reasons explained in (b).
d) ZAP-70 could have a mutation in its kinase domain that prevents kinase activity. This could be a mutation that prevents ATP binding, or one that prevents phosphorylation on the activation loop tyrosine. Alternatively, ZAP-70 could have a mutation in the linker region between the SH2 domains and the kinase domain that prevents phosphorylation of this linker region. In the absence of phosphorylation of this linker region, ZAP-70 remains in an auto-inhibited conformation, and would not phosphorylate its downstream substrates, ZAP-70 and LAT.
Unlike TCR signaling, B cell receptor (BCR) signaling is not initiated by a Src-family kinase phosphorylating tyrosine resides in ITAM motifs of BCR signaling subunits.
Second messengers, such as calcium ions (Ca2+), are chemical mediators commonly used in intracellular signaling pathways. Despite its common usage in many different cell types in the body, Ca2+ has specific effects in lymphocytes following antigen receptor stimulation. The specific responses of lymphocytes to increased concentrations of intracellular Ca2+ are determined by:
Immunoreceptor signaling proteins, such as the TCR chain and CD3 subunits, have conserved ITAM motifs in their cytoplasmic tails. When fully phosphorylated, the ITAM recruits a tyrosine kinase with a tandem SH2 domain structure at the amino-terminal end of the protein. Tandem SH2 domain-containing kinases do not bind to sequences in other proteins, even if they contain a phosphorylated tyrosine because:
The TCR signaling module leading to transcription factor activation is dependent on the enzyme phospholipase-C- (PLC- ). The mechanism by which PLC- activates multiple transcription factors is by:
TCR stimulation was shown to affect ICAM-1 (integrin ligand) binding to LFA-1 (integrin) on T cells. To demonstrate this, varying concentrations of purified ICAM-1 were added to unstimulated or TCR-stimulated T cells, and the amount of ICAM-1 binding was measured. The data from such an experiment are displayed on Figure. Assign the red or blue lines correctly to 'unstimulated' or 'TCR-stimulated' T cells, and explain the reasoning for your answer. 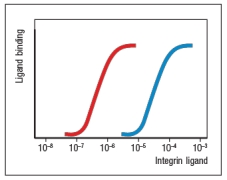
The TCR and BCR are multi-subunit receptor complexes. Experiments examining the synthesis and transport of these receptors to the lymphocyte cell surface have shown that the signaling subunits of each receptor complex are required for transport of the ligand-binding receptor subunits to the cell surface. One possible reason for this stringent control on cell surface expression is:
The immunosuppressive drug rapamycin acts by inhibiting mTOR. When activated T cells are treated with rapamycin in a cell culture assay, they show greatly diminished proliferation, and accumulate to much lower numbers than control-treated cells. This is because:
All of the modular protein domains used for signaling protein interactions bind to ligands that are transiently generated following receptor stimulation.
The integrin LFA-1 is constitutively expressed on the surface of resting T cells. Yet, integrin-dependent T cell adhesion to antigen-presenting cells increases substantially following TCR stimulation. This increased integrin-dependent adhesion is mediated in part by:
TCR and CD28 signaling together lead to maximal production of IL-2 by the activated T cell. Experiments investigating the mechanism underlying the CD28 co-stimulation-mediated increase in IL-2 production show that T cells stimulated through the TCR plus CD28 have increased levels of IL-2 mRNA compared to cells stimulated through the TCR alone. One important component contributing to increased IL-2 mRNA levels is:
An important transcription factor activated by antigen receptor signaling in lymphocytes is an NF B heterodimer of the two subunits, p50 and p65Rel. Defects in the I B-kinase complex (NEMO) or mutations in I B that prevent its phosphorylation interfere with NF B activation and result in severe immunodeficiency diseases. This is due to the important function of:
Human patients with genetic defects that result in a failure to produce the calcium channel protein ORAI1, or the ER calcium sensor protein STIM1, have severe immunodeficiency diseases. An immunosuppressive drug that would most closely mimic these primary immunodeficiencies is:
Small GTPases, such as Ras, Rho, and cdc42, are activated when they exchange their bound GDP for GTP. In the GTP-bound state, these proteins contribute to signaling by:
Using an antibody that recognizes the phosphorylated, but not the non-phosphorylated form of the transcription factor, NFAT, T cells are permeabilized, stained with this antibody, and analyzed by flow cytometry. Which of the data in Figure represent the expected pattern of staining from wild-type T cells before and after TCR stimulation. 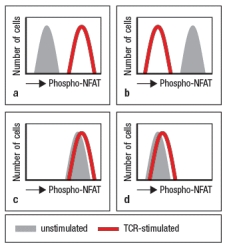
Humans with defective expression of the integrin LFA-1 have an immunodeficiency disease characterized by the failure of lymphocytes and granulocytes to migrate to tissues at sites of infection or inflammation. A similar immunodeficiency would be expected if individuals had mutations disrupting the gene for:
The TCR and BCR are each composed of two modules, an antigen-binding module and a signaling module; furthermore, in each case, the two functional modules are encoded by distinct polypeptides. In addition, the tyrosine kinases that initiate antigen receptor signaling are also separate proteins from those of each receptor. This is a different strategy for receptor signaling than the case of receptor tyrosine kinases, where the enzyme is an intrinsic component of the ligand-binding receptor protein. Name one advantage of this organization of the TCR and BCR that accounts for the expression of ZAP-70 and Syk, as well as ITAM-containing immunoreceptors, in many different subsets of immune cells.
T cells with defective TCR signaling are discovered, and found to have an inactivating mutation in a key TCR signaling protein. Using an antibody that recognizes the phosphorylated (activated) form of the Erk Map-kinase, stimulated T cells are permeabilized, stained with this antibody, and analyzed on the flow cytometer. These data are shown in Figure Q21). 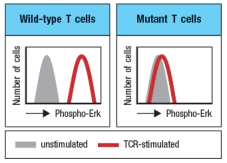 Figure Q21) Additional experiments examining Ca2+ influx into T cells following TCR stimulation show a normal response in the mutant T cells. One likely candidate gene that could be mutated in the defective cells is:
Figure Q21) Additional experiments examining Ca2+ influx into T cells following TCR stimulation show a normal response in the mutant T cells. One likely candidate gene that could be mutated in the defective cells is:
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)