Exam 15: Autoimmunity and Transplantation
All autoreactive CD4 T cells are not necessarily able to cause autoimmune diseases. Depending on the target tissue expressing the antigen recognized by the effector CD4 T cells, and the cytokines made by these effector T cells, autoimmune tissue damage may or may not occur.
True
Mutations in the intracellular sensor gene NOD2 are associated with Crohn's disease, a chronic severe inflammatory disease of the gastrointestinal tract. NOD2 is normally expressed in Paneth cells of the intestine, as well as in macrophages, monocytes and dendritic cells. To understand the effects of the NOD2 mutation, a line of knock-in mice was generated in which the wild-type NOD2 allele (NOD2+/+) was replaced with a variant disease-associated allele that causes a frameshift mutation in the NOD2 protein coding sequence (NOD2m/m). Isolated macrophages from wild-type mice were and those from NOD2m/m mice were stimulated in vitro with the NOD2 ligand, muramyl dipeptide (MDP, a component of bacterial peptidoglycan), and inflammatory cytokines IL-6 and TNF- were measured 3 hours later, as shown in Figure Q2)24). What is the most likely explanation for why this NOD2 mutation predisposes individuals to Crohn's disease? 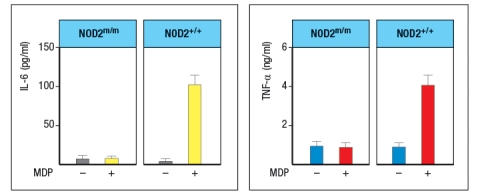
A defect in barrier immunity at the intestinal epithelium leads to increased exposure of adaptive immune cells to intestinal microbes. This increased exposure erodes the delicate balance of tolerogenic versus inflammatory responses to commensal microbes, resulting in increased effector responses to these organisms. In turn, this leads to chronic inflammation. Specifically, NOD2 is an intracellular receptor for the muramyl dipeptide derived from bacterial peptidoglycan, and its stimulation activates the transcription factor NF B and the expression of genes encoding pro-inflammatory cytokines and chemokines. In Paneth cells-specialized intestinal epithelial cells in the base of the small intestinal crypts-activation of NOD2 stimulates the release of granules containing antimicrobial peptides that help sequester commensal bacteria to the intestinal lumen, away from the adaptive immune system. Mutant forms of NOD2 that have lost this function limit this innate antibacterial response, thereby predisposing the individual to having heightened effector CD4 T-cell responses to the commensal microbiota and consequent chronic intestinal inflammation.
AIRE is a transcriptional regulator that promotes the expression of some 'tissue-specific' proteins in thymic stromal cells. This provides a means to induce central tolerance of developing T cells to these antigens. Patients with inactivating mutations in AIRE (a disease known as APECED) develop a range of symptoms, several of which involve autoimmune attack of exocrine glands. However, analysis of many patients with APECED reveals that some organs in the body are never attacked by autoimmune T cells in individuals with this syndrome, whereas other organs are commonly found to be destroyed in these patients. This targeted autoimmune response against a subset of tissues in APECED patients indicates:
B
Treg cells that express FoxP3 are generally thought to have T-cell receptors that recognize self-peptides bound to MHC class II molecules. In the skin, keratin and filaggrin are among the self-antigens expressed. FoxP3+ Treg cells found in skin and skin-draining lymph nodes might be specific for the self-antigen, filaggrin. These FoxP3+ filaggrin-specific Treg cells:
A subset of patients with imbalances in glucose metabolism are found to have autoantibodies to the insulin receptor. These patients, as well as patients with myasthenia gravis, may be treated with a procedure known as plasmapheresis. During plasmapheresis for these disorders, blood is removed from the patient, and then separated into two fractions, one containing cells, and the other containing the plasma. The plasma is then treated to deplete it of antibodies, and then the cells plus the antibody-depleted plasma are returned to the patient. This cumbersome treatment may be necessary because, for these diseases:
Immune privileged sites, such as the brain, the eye, and the testis, are often the targets of autoimmune attack. Thus, once effector T cells are generated that have specificity for autoantigens expressed in these tissues, the effector cells can gain entry to the tissue and cause tissue damage. However, under normal circumstances, the priming and differentiation of effector cells specific for antigens found in the brain, for example, is generally prevented. This is because 'immune privileged' sites:
A disease resembling systemic lupus erythematosis (SLE) can be induced in mice. A key characteristic of this disease is the production of autoantibodies with specificity for double-stranded DNA (dsDNA), nucleosomes, and ribonucleotide-protein complexes (RNPs). When these mice are treated with a small molecule TLR7 and TLR9 inhibitor, twice a week starting at 4 months of age, the data in Figure Q4) are obtained. 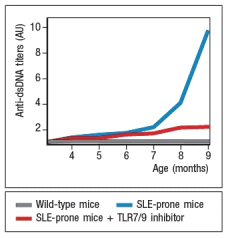 Figure Q4) In this system, the TLR7/TLR9 inhibitor:
Figure Q4) In this system, the TLR7/TLR9 inhibitor:
While relatively rare, individuals with a homozygous deficiency in complement C1 activity have been identified, and >90% of them developed a lupus-like illness at a very young age. These individuals had significant kidney damage and damage to blood vessels in the central nervous system, both of which were associated with severe inflammation. A reasonable hypothesis to explain the development of the lupus-like disease in these patients is:
A small subset of patients taking antibiotics such as minocycline develop symptoms resembling those of systemic lupus erythematosis (SLE). These symptoms include a severe skin rash that may cover the legs and trunk, arthritic joint pain, and swelling of the lower limbs, as well as the face, lips, ears, and tongue. These symptoms subside once the antibiotic is discontinued. In these individuals, a skin test for hypersensitivity to minocycline within 15 minutes of intradermal injection of the drug is negative. Analysis of the peripheral blood from these patients would likely reveal:
Nearly 80% of patients with autoimmune diabetes, also known as type 1 diabetes, have antibodies to the glutamic acid decarboxylase protein (GAD65) that is made by pancreatic -islet cells. Yet, the role of autoantibodies directed at GAD65 in the initiation of this disease is currently unclear. How might autoantibodies to GAD65 arise if they are not involved in the onset or initiation of type 1 diabetes?
Due to its multiple roles in promoting inflammatory responses, the complement system has been a target for the development of compounds that interfere with the complement system as a means of treating chronic autoimmune diseases. While some compounds inhibit the formation of the membrane attack complex, others are aimed at inhibiting earlier steps in the complement cascade. One such compound is an inhibitor of the C5a receptor, a receptor expressed on macrophages, dendritic cells, and granulocytes. The C5a receptor inhibitor would function to:
Multiple sclerosis is an autoimmune disease of the central nervous system that leads to demyelination of the nerves. Symptoms include tingling, numbness, muscle weakness, problems with coordination and balance, as well as other neurological problems. To understand more about this disease, a mouse model has been developed in which mice are experimentally induced to generate an autoimmune response to autoantigens expressed on the myelin sheaths of nerves in the central nervous system and spinal cord. To investigate the major immune system component responsible for the disease symptoms in EAE, lines of knockout mice were tested for the development of this autoimmune disease. To induce disease, mice are immunized with a peptide of murine myelin oligodendrocyte glycoprotein (MOG) in complete Freund's adjuvant (an adjuvant containing killed mycobacteria). Then disease symptoms are monitored on a scale of 1-5, where 1 is mild disease and 5 is moribundity and death. In comparison to wild-type mice, mice deficient in IFN- and mice deficient in IL-17 were each tested for development of EAE. The results are shown in Figure Q3)30)A. ![Multiple sclerosis is an autoimmune disease of the central nervous system that leads to demyelination of the nerves. Symptoms include tingling, numbness, muscle weakness, problems with coordination and balance, as well as other neurological problems. To understand more about this disease, a mouse model has been developed in which mice are experimentally induced to generate an autoimmune response to autoantigens expressed on the myelin sheaths of nerves in the central nervous system and spinal cord. To investigate the major immune system component responsible for the disease symptoms in EAE, lines of knockout mice were tested for the development of this autoimmune disease. To induce disease, mice are immunized with a peptide of murine myelin oligodendrocyte glycoprotein (MOG) in complete Freund's adjuvant (an adjuvant containing killed mycobacteria). Then disease symptoms are monitored on a scale of 1-5, where 1 is mild disease and 5 is moribundity and death. In comparison to wild-type mice, mice deficient in IFN- \gamma and mice deficient in IL-17 were each tested for development of EAE. The results are shown in Figure Q3)30)A. or IL-17-deficient mice were immunized with MOG peptide in CFA, and 10 days later, lymph node cells were isolated, and were stimulated for 3 days with MOG peptide. T cells were then isolated from the cultures of wild-type lymph node cells, as were the T cells from the Il17<sup>-/-</sup> cultures. Wild-type or Il17<sup>-/-</sup> T cells were then adoptively transferred into naive wild-type recipient mice, which were then monitored for EAE disease symptoms. The incidence of mice showing clinical disease is shown in Figure 3)30)B; the labels indicate the source of the transferred T cells. b) Do these data confirm or refute your answer to (a)? Explain your reasoning. c) What is a likely explanation for the results seen when EAE is induced in IFN- \gamma -deficient mice compared to wild-type and IL-17-deficient mice? Vitamin D and its metabolites have shown to provide partial protection against several autoimmune diseases in mice, including EAE. These findings correlate with the fact that multiple sclerosis incidence is reduced in areas of high sunlight exposure, and is also reduced in individuals on diets high in Vitamin D. To examine the mechanism of this protective effect of Vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)<sub>2</sub>D<sub>3</sub>], the active form of vitamin D in the body, was administered to mice starting at 2 weeks post-immunization with MOG+CFA. As a control, a second group of mice are given the vehicle used to dissolve the 1,25(OH)<sub>2</sub>D<sub>3</sub> on its own (vehicle control). 1,25(OH)<sub>2</sub>D<sub>3</sub> is known to act by binding to the vitamin D receptor (VDR), a protein that functions in transcriptional regulation. 1,25(OH)<sub>2</sub>D<sub>3</sub> enters cells, and binds to VDR. The activated VDR then migrates to the nucleus where it binds to DNA, functioning either as a transcriptional activator or as a repressor, depending on its binding partners. The results of 1,25(OH)<sub>2</sub>D<sub>3</sub> treatment on EAE induction are shown in Figure . In a second set of studies, naive CD4 T cells were isolated from wild-type mice, and were stimulated in vitro with anti-CD3/CD28 antibodies plus IL-6 and TGF- \beta for 3 days, and then expanded in IL-23 for an additional 4 days in the presence or absence of 1,25(OH)<sub>2</sub>D<sub>3</sub> or vehicle control. Following this, cells were restimulated with anti-CD3/CD28 antibodies, and 2 days later, supernatants were analyzed for IL-17 and IL-22, as shown in Figure. d) Given these data, propose a mechanism by which 1,25(OH)<sub>2</sub>D<sub>3</sub> is acting in the EAE disease model. Additional studies were performed in which mice were treated with 1,25(OH)<sub>2</sub>D<sub>3</sub> (or vehicle control) starting at the time of immunization with MOG + CFA, and then analyzed two weeks later. CD4 T cells from the spleen or the spinal cord were stained with antibodies to CD25 and the transcription factor FoxP3, and the results are displayed in Figure. e) Based on the data shown above, what might be another role for 1,25(OH)<sub>2</sub>D<sub>3</sub> that contributes to the ability of this vitamin D metabolite to inhibit autoimmune diseases like EAE or multiple sclerosis?](https://storage.examlex.com/TB1128/11eaa8f4_80d6_0e33_b44f_d551bfdbdeab_TB1128_00.jpg) or IL-17-deficient mice were immunized with MOG peptide in CFA, and 10 days later, lymph node cells were isolated, and were stimulated for 3 days with MOG peptide. T cells were then isolated from the cultures of wild-type lymph node cells, as were the T cells from the Il17-/- cultures. Wild-type or Il17-/- T cells were then adoptively transferred into naive wild-type recipient mice, which were then monitored for EAE disease symptoms. The incidence of mice showing clinical disease is shown in Figure 3)30)B; the labels indicate the source of the transferred T cells.
or IL-17-deficient mice were immunized with MOG peptide in CFA, and 10 days later, lymph node cells were isolated, and were stimulated for 3 days with MOG peptide. T cells were then isolated from the cultures of wild-type lymph node cells, as were the T cells from the Il17-/- cultures. Wild-type or Il17-/- T cells were then adoptively transferred into naive wild-type recipient mice, which were then monitored for EAE disease symptoms. The incidence of mice showing clinical disease is shown in Figure 3)30)B; the labels indicate the source of the transferred T cells. ![Multiple sclerosis is an autoimmune disease of the central nervous system that leads to demyelination of the nerves. Symptoms include tingling, numbness, muscle weakness, problems with coordination and balance, as well as other neurological problems. To understand more about this disease, a mouse model has been developed in which mice are experimentally induced to generate an autoimmune response to autoantigens expressed on the myelin sheaths of nerves in the central nervous system and spinal cord. To investigate the major immune system component responsible for the disease symptoms in EAE, lines of knockout mice were tested for the development of this autoimmune disease. To induce disease, mice are immunized with a peptide of murine myelin oligodendrocyte glycoprotein (MOG) in complete Freund's adjuvant (an adjuvant containing killed mycobacteria). Then disease symptoms are monitored on a scale of 1-5, where 1 is mild disease and 5 is moribundity and death. In comparison to wild-type mice, mice deficient in IFN- \gamma and mice deficient in IL-17 were each tested for development of EAE. The results are shown in Figure Q3)30)A. or IL-17-deficient mice were immunized with MOG peptide in CFA, and 10 days later, lymph node cells were isolated, and were stimulated for 3 days with MOG peptide. T cells were then isolated from the cultures of wild-type lymph node cells, as were the T cells from the Il17<sup>-/-</sup> cultures. Wild-type or Il17<sup>-/-</sup> T cells were then adoptively transferred into naive wild-type recipient mice, which were then monitored for EAE disease symptoms. The incidence of mice showing clinical disease is shown in Figure 3)30)B; the labels indicate the source of the transferred T cells. b) Do these data confirm or refute your answer to (a)? Explain your reasoning. c) What is a likely explanation for the results seen when EAE is induced in IFN- \gamma -deficient mice compared to wild-type and IL-17-deficient mice? Vitamin D and its metabolites have shown to provide partial protection against several autoimmune diseases in mice, including EAE. These findings correlate with the fact that multiple sclerosis incidence is reduced in areas of high sunlight exposure, and is also reduced in individuals on diets high in Vitamin D. To examine the mechanism of this protective effect of Vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)<sub>2</sub>D<sub>3</sub>], the active form of vitamin D in the body, was administered to mice starting at 2 weeks post-immunization with MOG+CFA. As a control, a second group of mice are given the vehicle used to dissolve the 1,25(OH)<sub>2</sub>D<sub>3</sub> on its own (vehicle control). 1,25(OH)<sub>2</sub>D<sub>3</sub> is known to act by binding to the vitamin D receptor (VDR), a protein that functions in transcriptional regulation. 1,25(OH)<sub>2</sub>D<sub>3</sub> enters cells, and binds to VDR. The activated VDR then migrates to the nucleus where it binds to DNA, functioning either as a transcriptional activator or as a repressor, depending on its binding partners. The results of 1,25(OH)<sub>2</sub>D<sub>3</sub> treatment on EAE induction are shown in Figure . In a second set of studies, naive CD4 T cells were isolated from wild-type mice, and were stimulated in vitro with anti-CD3/CD28 antibodies plus IL-6 and TGF- \beta for 3 days, and then expanded in IL-23 for an additional 4 days in the presence or absence of 1,25(OH)<sub>2</sub>D<sub>3</sub> or vehicle control. Following this, cells were restimulated with anti-CD3/CD28 antibodies, and 2 days later, supernatants were analyzed for IL-17 and IL-22, as shown in Figure. d) Given these data, propose a mechanism by which 1,25(OH)<sub>2</sub>D<sub>3</sub> is acting in the EAE disease model. Additional studies were performed in which mice were treated with 1,25(OH)<sub>2</sub>D<sub>3</sub> (or vehicle control) starting at the time of immunization with MOG + CFA, and then analyzed two weeks later. CD4 T cells from the spleen or the spinal cord were stained with antibodies to CD25 and the transcription factor FoxP3, and the results are displayed in Figure. e) Based on the data shown above, what might be another role for 1,25(OH)<sub>2</sub>D<sub>3</sub> that contributes to the ability of this vitamin D metabolite to inhibit autoimmune diseases like EAE or multiple sclerosis?](https://storage.examlex.com/TB1128/11eaa8f4_80d6_0e34_b44f_35a869a3a7d1_TB1128_00.jpg) b) Do these data confirm or refute your answer to (a)? Explain your reasoning.
c) What is a likely explanation for the results seen when EAE is induced in IFN- -deficient mice compared to wild-type and IL-17-deficient mice?
Vitamin D and its metabolites have shown to provide partial protection against several autoimmune diseases in mice, including EAE. These findings correlate with the fact that multiple sclerosis incidence is reduced in areas of high sunlight exposure, and is also reduced in individuals on diets high in Vitamin
D. To examine the mechanism of this protective effect of Vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the active form of vitamin D in the body, was administered to mice starting at 2 weeks post-immunization with MOG+CFA. As a control, a second group of mice are given the vehicle used to dissolve the 1,25(OH)2D3 on its own (vehicle control). 1,25(OH)2D3 is known to act by binding to the vitamin D receptor (VDR), a protein that functions in transcriptional regulation. 1,25(OH)2D3 enters cells, and binds to VDR. The activated VDR then migrates to the nucleus where it binds to DNA, functioning either as a transcriptional activator or as a repressor, depending on its binding partners. The results of 1,25(OH)2D3 treatment on EAE induction are shown in Figure .
b) Do these data confirm or refute your answer to (a)? Explain your reasoning.
c) What is a likely explanation for the results seen when EAE is induced in IFN- -deficient mice compared to wild-type and IL-17-deficient mice?
Vitamin D and its metabolites have shown to provide partial protection against several autoimmune diseases in mice, including EAE. These findings correlate with the fact that multiple sclerosis incidence is reduced in areas of high sunlight exposure, and is also reduced in individuals on diets high in Vitamin
D. To examine the mechanism of this protective effect of Vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the active form of vitamin D in the body, was administered to mice starting at 2 weeks post-immunization with MOG+CFA. As a control, a second group of mice are given the vehicle used to dissolve the 1,25(OH)2D3 on its own (vehicle control). 1,25(OH)2D3 is known to act by binding to the vitamin D receptor (VDR), a protein that functions in transcriptional regulation. 1,25(OH)2D3 enters cells, and binds to VDR. The activated VDR then migrates to the nucleus where it binds to DNA, functioning either as a transcriptional activator or as a repressor, depending on its binding partners. The results of 1,25(OH)2D3 treatment on EAE induction are shown in Figure .
![Multiple sclerosis is an autoimmune disease of the central nervous system that leads to demyelination of the nerves. Symptoms include tingling, numbness, muscle weakness, problems with coordination and balance, as well as other neurological problems. To understand more about this disease, a mouse model has been developed in which mice are experimentally induced to generate an autoimmune response to autoantigens expressed on the myelin sheaths of nerves in the central nervous system and spinal cord. To investigate the major immune system component responsible for the disease symptoms in EAE, lines of knockout mice were tested for the development of this autoimmune disease. To induce disease, mice are immunized with a peptide of murine myelin oligodendrocyte glycoprotein (MOG) in complete Freund's adjuvant (an adjuvant containing killed mycobacteria). Then disease symptoms are monitored on a scale of 1-5, where 1 is mild disease and 5 is moribundity and death. In comparison to wild-type mice, mice deficient in IFN- \gamma and mice deficient in IL-17 were each tested for development of EAE. The results are shown in Figure Q3)30)A. or IL-17-deficient mice were immunized with MOG peptide in CFA, and 10 days later, lymph node cells were isolated, and were stimulated for 3 days with MOG peptide. T cells were then isolated from the cultures of wild-type lymph node cells, as were the T cells from the Il17<sup>-/-</sup> cultures. Wild-type or Il17<sup>-/-</sup> T cells were then adoptively transferred into naive wild-type recipient mice, which were then monitored for EAE disease symptoms. The incidence of mice showing clinical disease is shown in Figure 3)30)B; the labels indicate the source of the transferred T cells. b) Do these data confirm or refute your answer to (a)? Explain your reasoning. c) What is a likely explanation for the results seen when EAE is induced in IFN- \gamma -deficient mice compared to wild-type and IL-17-deficient mice? Vitamin D and its metabolites have shown to provide partial protection against several autoimmune diseases in mice, including EAE. These findings correlate with the fact that multiple sclerosis incidence is reduced in areas of high sunlight exposure, and is also reduced in individuals on diets high in Vitamin D. To examine the mechanism of this protective effect of Vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)<sub>2</sub>D<sub>3</sub>], the active form of vitamin D in the body, was administered to mice starting at 2 weeks post-immunization with MOG+CFA. As a control, a second group of mice are given the vehicle used to dissolve the 1,25(OH)<sub>2</sub>D<sub>3</sub> on its own (vehicle control). 1,25(OH)<sub>2</sub>D<sub>3</sub> is known to act by binding to the vitamin D receptor (VDR), a protein that functions in transcriptional regulation. 1,25(OH)<sub>2</sub>D<sub>3</sub> enters cells, and binds to VDR. The activated VDR then migrates to the nucleus where it binds to DNA, functioning either as a transcriptional activator or as a repressor, depending on its binding partners. The results of 1,25(OH)<sub>2</sub>D<sub>3</sub> treatment on EAE induction are shown in Figure . In a second set of studies, naive CD4 T cells were isolated from wild-type mice, and were stimulated in vitro with anti-CD3/CD28 antibodies plus IL-6 and TGF- \beta for 3 days, and then expanded in IL-23 for an additional 4 days in the presence or absence of 1,25(OH)<sub>2</sub>D<sub>3</sub> or vehicle control. Following this, cells were restimulated with anti-CD3/CD28 antibodies, and 2 days later, supernatants were analyzed for IL-17 and IL-22, as shown in Figure. d) Given these data, propose a mechanism by which 1,25(OH)<sub>2</sub>D<sub>3</sub> is acting in the EAE disease model. Additional studies were performed in which mice were treated with 1,25(OH)<sub>2</sub>D<sub>3</sub> (or vehicle control) starting at the time of immunization with MOG + CFA, and then analyzed two weeks later. CD4 T cells from the spleen or the spinal cord were stained with antibodies to CD25 and the transcription factor FoxP3, and the results are displayed in Figure. e) Based on the data shown above, what might be another role for 1,25(OH)<sub>2</sub>D<sub>3</sub> that contributes to the ability of this vitamin D metabolite to inhibit autoimmune diseases like EAE or multiple sclerosis?](https://storage.examlex.com/TB1128/11eaa8f4_80d6_3545_b44f_99b12fc2a040_TB1128_00.jpg) In a second set of studies, naive CD4 T cells were isolated from wild-type mice, and were stimulated in vitro with anti-CD3/CD28 antibodies plus IL-6 and TGF- for 3 days, and then expanded in IL-23 for an additional 4 days in the presence or absence of 1,25(OH)2D3 or vehicle control. Following this, cells were restimulated with anti-CD3/CD28 antibodies, and 2 days later, supernatants were analyzed for IL-17 and IL-22, as shown in Figure.
In a second set of studies, naive CD4 T cells were isolated from wild-type mice, and were stimulated in vitro with anti-CD3/CD28 antibodies plus IL-6 and TGF- for 3 days, and then expanded in IL-23 for an additional 4 days in the presence or absence of 1,25(OH)2D3 or vehicle control. Following this, cells were restimulated with anti-CD3/CD28 antibodies, and 2 days later, supernatants were analyzed for IL-17 and IL-22, as shown in Figure.
![Multiple sclerosis is an autoimmune disease of the central nervous system that leads to demyelination of the nerves. Symptoms include tingling, numbness, muscle weakness, problems with coordination and balance, as well as other neurological problems. To understand more about this disease, a mouse model has been developed in which mice are experimentally induced to generate an autoimmune response to autoantigens expressed on the myelin sheaths of nerves in the central nervous system and spinal cord. To investigate the major immune system component responsible for the disease symptoms in EAE, lines of knockout mice were tested for the development of this autoimmune disease. To induce disease, mice are immunized with a peptide of murine myelin oligodendrocyte glycoprotein (MOG) in complete Freund's adjuvant (an adjuvant containing killed mycobacteria). Then disease symptoms are monitored on a scale of 1-5, where 1 is mild disease and 5 is moribundity and death. In comparison to wild-type mice, mice deficient in IFN- \gamma and mice deficient in IL-17 were each tested for development of EAE. The results are shown in Figure Q3)30)A. or IL-17-deficient mice were immunized with MOG peptide in CFA, and 10 days later, lymph node cells were isolated, and were stimulated for 3 days with MOG peptide. T cells were then isolated from the cultures of wild-type lymph node cells, as were the T cells from the Il17<sup>-/-</sup> cultures. Wild-type or Il17<sup>-/-</sup> T cells were then adoptively transferred into naive wild-type recipient mice, which were then monitored for EAE disease symptoms. The incidence of mice showing clinical disease is shown in Figure 3)30)B; the labels indicate the source of the transferred T cells. b) Do these data confirm or refute your answer to (a)? Explain your reasoning. c) What is a likely explanation for the results seen when EAE is induced in IFN- \gamma -deficient mice compared to wild-type and IL-17-deficient mice? Vitamin D and its metabolites have shown to provide partial protection against several autoimmune diseases in mice, including EAE. These findings correlate with the fact that multiple sclerosis incidence is reduced in areas of high sunlight exposure, and is also reduced in individuals on diets high in Vitamin D. To examine the mechanism of this protective effect of Vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)<sub>2</sub>D<sub>3</sub>], the active form of vitamin D in the body, was administered to mice starting at 2 weeks post-immunization with MOG+CFA. As a control, a second group of mice are given the vehicle used to dissolve the 1,25(OH)<sub>2</sub>D<sub>3</sub> on its own (vehicle control). 1,25(OH)<sub>2</sub>D<sub>3</sub> is known to act by binding to the vitamin D receptor (VDR), a protein that functions in transcriptional regulation. 1,25(OH)<sub>2</sub>D<sub>3</sub> enters cells, and binds to VDR. The activated VDR then migrates to the nucleus where it binds to DNA, functioning either as a transcriptional activator or as a repressor, depending on its binding partners. The results of 1,25(OH)<sub>2</sub>D<sub>3</sub> treatment on EAE induction are shown in Figure . In a second set of studies, naive CD4 T cells were isolated from wild-type mice, and were stimulated in vitro with anti-CD3/CD28 antibodies plus IL-6 and TGF- \beta for 3 days, and then expanded in IL-23 for an additional 4 days in the presence or absence of 1,25(OH)<sub>2</sub>D<sub>3</sub> or vehicle control. Following this, cells were restimulated with anti-CD3/CD28 antibodies, and 2 days later, supernatants were analyzed for IL-17 and IL-22, as shown in Figure. d) Given these data, propose a mechanism by which 1,25(OH)<sub>2</sub>D<sub>3</sub> is acting in the EAE disease model. Additional studies were performed in which mice were treated with 1,25(OH)<sub>2</sub>D<sub>3</sub> (or vehicle control) starting at the time of immunization with MOG + CFA, and then analyzed two weeks later. CD4 T cells from the spleen or the spinal cord were stained with antibodies to CD25 and the transcription factor FoxP3, and the results are displayed in Figure. e) Based on the data shown above, what might be another role for 1,25(OH)<sub>2</sub>D<sub>3</sub> that contributes to the ability of this vitamin D metabolite to inhibit autoimmune diseases like EAE or multiple sclerosis?](https://storage.examlex.com/TB1128/11eaa8f4_80d6_3546_b44f_839d05512cde_TB1128_00.jpg) d) Given these data, propose a mechanism by which 1,25(OH)2D3 is acting in the EAE disease model.
Additional studies were performed in which mice were treated with 1,25(OH)2D3 (or vehicle control) starting at the time of immunization with MOG + CFA, and then analyzed two weeks later. CD4 T cells from the spleen or the spinal cord were stained with antibodies to CD25 and the transcription factor FoxP3, and the results are displayed in Figure.
d) Given these data, propose a mechanism by which 1,25(OH)2D3 is acting in the EAE disease model.
Additional studies were performed in which mice were treated with 1,25(OH)2D3 (or vehicle control) starting at the time of immunization with MOG + CFA, and then analyzed two weeks later. CD4 T cells from the spleen or the spinal cord were stained with antibodies to CD25 and the transcription factor FoxP3, and the results are displayed in Figure.
![Multiple sclerosis is an autoimmune disease of the central nervous system that leads to demyelination of the nerves. Symptoms include tingling, numbness, muscle weakness, problems with coordination and balance, as well as other neurological problems. To understand more about this disease, a mouse model has been developed in which mice are experimentally induced to generate an autoimmune response to autoantigens expressed on the myelin sheaths of nerves in the central nervous system and spinal cord. To investigate the major immune system component responsible for the disease symptoms in EAE, lines of knockout mice were tested for the development of this autoimmune disease. To induce disease, mice are immunized with a peptide of murine myelin oligodendrocyte glycoprotein (MOG) in complete Freund's adjuvant (an adjuvant containing killed mycobacteria). Then disease symptoms are monitored on a scale of 1-5, where 1 is mild disease and 5 is moribundity and death. In comparison to wild-type mice, mice deficient in IFN- \gamma and mice deficient in IL-17 were each tested for development of EAE. The results are shown in Figure Q3)30)A. or IL-17-deficient mice were immunized with MOG peptide in CFA, and 10 days later, lymph node cells were isolated, and were stimulated for 3 days with MOG peptide. T cells were then isolated from the cultures of wild-type lymph node cells, as were the T cells from the Il17<sup>-/-</sup> cultures. Wild-type or Il17<sup>-/-</sup> T cells were then adoptively transferred into naive wild-type recipient mice, which were then monitored for EAE disease symptoms. The incidence of mice showing clinical disease is shown in Figure 3)30)B; the labels indicate the source of the transferred T cells. b) Do these data confirm or refute your answer to (a)? Explain your reasoning. c) What is a likely explanation for the results seen when EAE is induced in IFN- \gamma -deficient mice compared to wild-type and IL-17-deficient mice? Vitamin D and its metabolites have shown to provide partial protection against several autoimmune diseases in mice, including EAE. These findings correlate with the fact that multiple sclerosis incidence is reduced in areas of high sunlight exposure, and is also reduced in individuals on diets high in Vitamin D. To examine the mechanism of this protective effect of Vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)<sub>2</sub>D<sub>3</sub>], the active form of vitamin D in the body, was administered to mice starting at 2 weeks post-immunization with MOG+CFA. As a control, a second group of mice are given the vehicle used to dissolve the 1,25(OH)<sub>2</sub>D<sub>3</sub> on its own (vehicle control). 1,25(OH)<sub>2</sub>D<sub>3</sub> is known to act by binding to the vitamin D receptor (VDR), a protein that functions in transcriptional regulation. 1,25(OH)<sub>2</sub>D<sub>3</sub> enters cells, and binds to VDR. The activated VDR then migrates to the nucleus where it binds to DNA, functioning either as a transcriptional activator or as a repressor, depending on its binding partners. The results of 1,25(OH)<sub>2</sub>D<sub>3</sub> treatment on EAE induction are shown in Figure . In a second set of studies, naive CD4 T cells were isolated from wild-type mice, and were stimulated in vitro with anti-CD3/CD28 antibodies plus IL-6 and TGF- \beta for 3 days, and then expanded in IL-23 for an additional 4 days in the presence or absence of 1,25(OH)<sub>2</sub>D<sub>3</sub> or vehicle control. Following this, cells were restimulated with anti-CD3/CD28 antibodies, and 2 days later, supernatants were analyzed for IL-17 and IL-22, as shown in Figure. d) Given these data, propose a mechanism by which 1,25(OH)<sub>2</sub>D<sub>3</sub> is acting in the EAE disease model. Additional studies were performed in which mice were treated with 1,25(OH)<sub>2</sub>D<sub>3</sub> (or vehicle control) starting at the time of immunization with MOG + CFA, and then analyzed two weeks later. CD4 T cells from the spleen or the spinal cord were stained with antibodies to CD25 and the transcription factor FoxP3, and the results are displayed in Figure. e) Based on the data shown above, what might be another role for 1,25(OH)<sub>2</sub>D<sub>3</sub> that contributes to the ability of this vitamin D metabolite to inhibit autoimmune diseases like EAE or multiple sclerosis?](https://storage.examlex.com/TB1128/11eaa8f4_80d6_3547_b44f_257e3eff8e57_TB1128_00.jpg) e) Based on the data shown above, what might be another role for 1,25(OH)2D3 that contributes to the ability of this vitamin D metabolite to inhibit autoimmune diseases like EAE or multiple sclerosis?
e) Based on the data shown above, what might be another role for 1,25(OH)2D3 that contributes to the ability of this vitamin D metabolite to inhibit autoimmune diseases like EAE or multiple sclerosis?
Multiple mechanisms provide a series of checkpoints that function to maintain immunological self-tolerance. A breakdown in any one of these mechanisms is likely to lead to autoimmunity.
23) Multiple autoimmune diseases are associated with particular alleles of MHC class II molecules. For example, individuals expressing HLA-DR2 are nearly 16 times more likely to develop Goodpasture's syndrome than individuals expressing other HLA-DR alleles. The evidence to date indicate that these associations between MHC class II alleles and specific autoimmune diseases are due to:
All-trans retinoic acid (tRA) is a metabolite generated from vitamin A in the diet, and is a key component in generating an appropriate balance of FoxP3+ regulatory CD4 T cells versus TH17 effector CD4 T cells in mucosal tissues. Patients with some autoimmune diseases, such as psoriasis, derive benefit from oral synthetic retinoid treatment for their condition. This indicates:
26) Epidemiological evidence has indicated that exposure to particular infections can predispose individuals to specific autoimmune diseases. An example is the data linking Coxsackie virus infections with subsequent development of type 1 diabetes. In many cases, there is no indication of cross-reactive antigens between the pathogen and the autoantigens targeted by the autoimmune disease.
During inflammation, some proteins undergo modification, converting arginine residues in their sequences to citrulline. In smokers, an increased level of protein citrullination has been detected in cells of the lung, an observation that has been proposed as one explanation for the strong association between smoking and the autoimmune disease, rheumatoid arthritis. To determine a potential mechanism by which protein citrullination might lead to autoimmunity, studies were performed in mice. These mice were transgenic for the human MHC class II molecule, HLA DR4, the allele showing strong association with rheumatoid arthritis in humans. These mice were immunized with a peptide derived from the self-antigen vimentin (Vim65-77), or with a variant Vim65-77 peptide in which the arginine at position 70 of this peptide was converted to citrulline (Vim65-77(R70Cit)). Ten days later, T cells were isolated from draining lymph nodes of the immunized mice, and were stimulated in vitro with APCs plus each vimentin peptide. After three days of culture, IFN- levels in the supernatants of the cultures were measured, as shown in Figure Q1)18). To confirm that the T cell responses were directed at vimentin peptides bound to DR4, duplicate in vitro cultures received anti-DR4 blocking antibody. What hypothesis is suggested by these data to account for how protein citrullination might lead to autoimmunity? 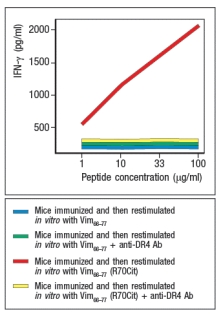
22) The majority of monogenic defects in humans that cause autoimmune diseases are in genes that regulate T cell responses. These include the AIRE, CTLA4, FOXP3, and FAS genes. These findings indicate that B cells and innate immune cells are not important in autoimmunity.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)