Exam 5: Atomic Theory: the Nuclear Model of the Atom
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
Which of the following is not one of the main features of Dalton's atomic theory?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
Which of the three major subatomic particles has/have a mass of approximately 1 u?
(i)e-
(ii)p+
(iii)n0
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
E
Which of the following is correct for the fourth period element in Group 3A/13?
Z Chemical symbol Atomic mass
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
A
One of the main features of Dalton's atomic theory no longer considered valid is: atoms cannot be created or destroyed.Which of the following is the best explanation of why this feature is no longer considered valid?
(Multiple Choice)
5.0/5  (39)
(39)
The nuclear symbol of an isotope of beryllium is 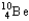 .Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.
Number of protons Number of neutrons Atomic number Mass number
.Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.
Number of protons Number of neutrons Atomic number Mass number
(Multiple Choice)
4.8/5  (41)
(41)
Which of the three major subatomic particles has/have a charge of -1?
(i)electron
(ii)proton
(iii)neutron
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is correct for the element with Z = 9?
Symbol Atomic mass Period Group
(Multiple Choice)
4.9/5  (31)
(31)
One of the features of Dalton's atomic theory no longer considered to be correct is: All atoms of each element are identical in every respect.Which of the following is the best explanation of why this feature is no longer considered valid?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following statements is/are false?
(i)Groups are arranged vertically in the periodic table
(ii)Periods are arranged horizontally in the periodic table
(iii)Periods are numbered from top to bottom
(iv)There are eight elements in the first period of the periodic table
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following is correct for the element with atomic number 20?
Symbol Atomic mass (u)Period Group
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following masses is closest to the mass of one atomic mass unit?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following elements are correctly matched with their period and group?
(i)C
First period
Group 4A/4
(ii)Ne
Second period
Group 8A/18
(iii)Al
Third period
Group 3A/13
(iv)Ca
Third period
Group 2A/2
(v)Br
Fourth period
Group 8A/18
(Multiple Choice)
4.8/5  (46)
(46)
The nuclear symbol of an isotope of potassium is 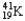 .Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.
Number of protons Number of neutrons Atomic number Mass number
.Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.
Number of protons Number of neutrons Atomic number Mass number
(Multiple Choice)
4.8/5  (41)
(41)
Consider the following model of a generic atom. 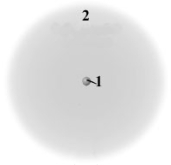 Which of the following is correct regarding the "regions" labeled 1 and 2?
Which of the following is correct regarding the "regions" labeled 1 and 2?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is a correct statement about the Rutherford scattering experiment?
(Multiple Choice)
4.9/5  (39)
(39)
Calculate the atomic mass of the element having the following distribution of isotopes:
5)8% 53.9396 u
91)8% 55.9349 u
2)1% 56.9354 u
0)3% 57.9333 u
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following statements about isotopes is/are correct?
(i)They have different masses
(ii)They have different numbers of neutrons
(iii)They have different electrical charges
(iv)They have different nuclear symbols
(v)They have different mass numbers
(Multiple Choice)
4.8/5  (33)
(33)
The chemical symbol of argon is Ar.An isotope of this element has an atomic number of 18 and a mass number of 38.What are the nuclear symbol,the number of protons,and the number of neutrons of this isotope of argon?
Nuclear symbol Number of protons Number of neutrons
(Multiple Choice)
4.7/5  (38)
(38)
Showing 1 - 20 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)