Exam 19: Oxidation-Reduction Redoxreactions
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
In balancing the half-reaction
SO42-(aq) 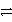 S(s)how many H+ ions are added to which side?
S(s)how many H+ ions are added to which side?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
D
When zinc is plated to iron,the zinc corrodes rather than the iron.Which statement is true?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
Identify the reduction half-reaction in the redox equation:
4 Ag + O2(g)+ 2 H2O 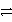 4 Ag+ + 4 OH-
4 Ag+ + 4 OH-
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
D
In the reaction of carbon with oxygen,which substance is oxidized? The equation for the reaction is
C(s)+ O2(g) 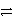 CO2(g).
CO2(g).
(Multiple Choice)
4.8/5  (35)
(35)
What is the oxidizing agent in this redox reaction?
2 MnO2(s)+ 2 NH4Cl(s)+ Zn(s) 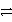 Zn(NH3)2Cl2(s)+ Mn2O3(s)+ H2O(
Zn(NH3)2Cl2(s)+ Mn2O3(s)+ H2O( 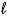 )
)
(Multiple Choice)
4.8/5  (34)
(34)
Which among the following are the parts that comprose an electrolytic cell?
(i)Two electrodes
(ii)A molecular liquid
(iii)A salt bridge
(iv)An ionic solution
(Multiple Choice)
4.7/5  (40)
(40)
Consider the following beakers.In the beaker on the left copper metal is placed in a solution of silver nitrate.The reaction is allowed to run for 60 minutes producing the products shown on the right. 
 The equation for the oxidation half-reaction would be:
The equation for the oxidation half-reaction would be:
(Multiple Choice)
4.8/5  (36)
(36)
What is the reducing agent in the reaction of hydrazine as a rocket fuel: N2H4( 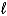 )+ O2(g)
)+ O2(g) 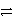 N2(g)+ 2 H2O(
N2(g)+ 2 H2O( 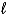 )?
)?
(Multiple Choice)
4.9/5  (35)
(35)
What is the oxidation number of sulfur in sulfite ion,SO32-?
(Multiple Choice)
4.9/5  (39)
(39)
Consider the following table of relative strengths of oxidizing and reducing agents:
Oxidizing Agent
Reducing Agent
Stronger
A+ + e- 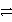 A
Weaker
B2+ + 2 e-
A
Weaker
B2+ + 2 e- 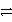 B
Weaker
C3+ + 3 e-
B
Weaker
C3+ + 3 e- 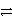 C
Stronger
Which correctly lists the oxidizing agents in order of increasing strength?
C
Stronger
Which correctly lists the oxidizing agents in order of increasing strength?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following is the half-reaction for reduction of chlorine to chloride ions in water?
(Multiple Choice)
4.8/5  (36)
(36)
Examine the two beakers shown below. 
 If the metals were connected by a wire containing a meter and the two solutions by a salt bridge,the meter shows are reading of 0.95 V.Which of the following is correct?
If the metals were connected by a wire containing a meter and the two solutions by a salt bridge,the meter shows are reading of 0.95 V.Which of the following is correct?
(Multiple Choice)
4.9/5  (36)
(36)
Which substance gets reduced in this redox reaction?
Pb(s)+ PbO2(s)+ 2 H2SO4(aq) 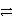 2 PbSO4(s)+ 2 H2O(
2 PbSO4(s)+ 2 H2O( 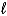 )
)
(Multiple Choice)
4.9/5  (30)
(30)
Consider the following table of relative strengths of oxidizing and reducing agents:
Oxidizing Agent
Reducing Agent
Stronger
A+ + e- 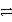 A
Weaker
B2+ + 2 e-
A
Weaker
B2+ + 2 e- 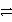 B
Weaker
C3+ + 3 e-
B
Weaker
C3+ + 3 e- 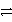 C
Stronger
Which of the following is the correct net ionic equation for the redox reaction between A+ and C and the correct direction that is favored?
C
Stronger
Which of the following is the correct net ionic equation for the redox reaction between A+ and C and the correct direction that is favored?
(Multiple Choice)
4.8/5  (41)
(41)
How many electrons are transferred in the balanced redox equation that results from combining these two half-reactions on a molar scale?
Al 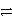 Al3+ + 3e- and Cu2+ + 2 e-
Al3+ + 3e- and Cu2+ + 2 e- 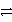 Cu
Cu
(Multiple Choice)
4.9/5  (33)
(33)
Identify the oxidation half-reaction in the following group.
(Multiple Choice)
4.7/5  (32)
(32)
What is the balanced redox equation that results from the combination of the following half-reactions?
Cr3+ + 3e- 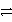 Cr and 2 Cl-
Cr and 2 Cl- 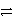 Cl2 + 2 e-
Cl2 + 2 e-
(Multiple Choice)
4.7/5  (38)
(38)
What role does the reducing agent play in a redox reaction?
(Multiple Choice)
4.9/5  (43)
(43)
Showing 1 - 20 of 44
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)